Global Blood-based Biomarker For Alzheimers Disease Diagnostics Market
Global Blood-based Biomarker For Alzheimers Disease Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Type (Amyloid-related Markers, Tau-related Markers, Neurodegeneration, Others), By Technology (Immunoassays, Mass spectrometry-based assays, Next-generation platforms, Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Report Overview
Table of Contents
Blood-based Biomarker For Alzheimer’s Disease Diagnostics Market Summary
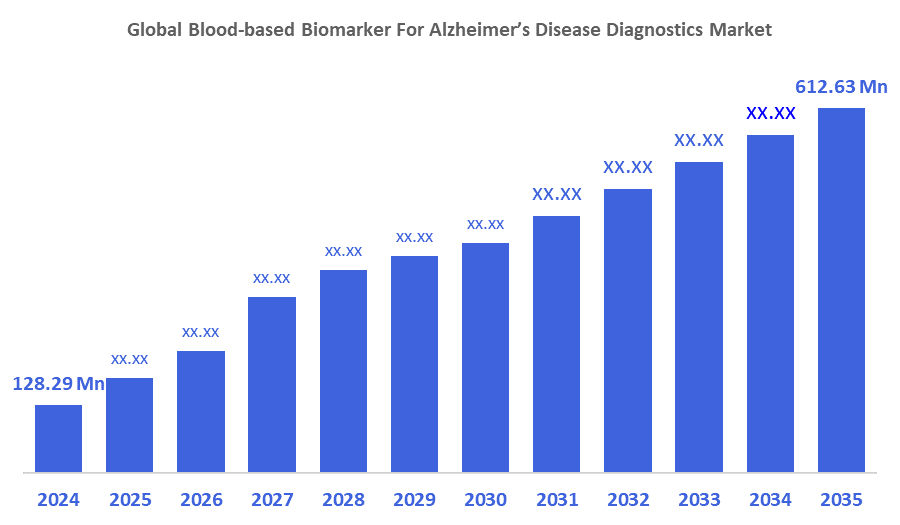
The Global Blood-Based Biomarker For Alzheimer’s Disease Diagnostics Market Size Was Estimated at USD 128.29 Million in 2024 and is Expected to Reach USD 612.63 Million by 2035, growing at a CAGR of 15.27% from 2025 to 2035. The market for blood-based Alzheimer's diagnostics is expanding because of the rising incidence of Alzheimer's disease, the urgent need for non-invasive, affordable early diagnosis, improvements in technology that increase accessibility and accuracy, and regulatory approvals for new assays.
Key Regional and Segment-Wise Insights
- In 2024, North America held the greatest revenue share of 46.53% in the global market and led the blood-based biomarkers for Alzheimer's disease diagnostics market.
- In 2024, the tau-related markers segment held the biggest revenue share of 36.4% and dominated the global market based on type.
- In 2024, the immunoassays segment had the highest revenue share of 52.62% based on technology.
Global Market Forecast and Revenue Outlook
- 2024 Market Size: USD 128.29 Million
- 2035 Projected Market Size: USD 612.63 Million
- CAGR (2025-2035): 15.27%
- North America: Largest market in 2024
The Blood-based biomarkers for Alzheimer's disease diagnostics market represents a healthcare industry segment dedicated to developing blood tests that detect biological markers indicative of Alzheimer's disease. The biomarkers, including tau proteins, amyloid-beta, and neurofilament light chain (NfL), enable earlier diagnosis than standard cerebrospinal fluid (CSF) testing or neuroimaging and provide less invasive diagnostic methods. Various factors drive this market forward, including population aging as well as increasing global Alzheimer's disease prevalence, along with requirements for earlier, precise diagnostic methods and growing patient healthcare professional knowledge. Blood-based diagnostics gain momentum because they present affordable alternatives to existing expensive diagnostic methods, which also involve intrusive procedures.
The technological advancements in blood-based biomarker diagnostics have significantly expanded their diagnostic capabilities. Modern ultrasensitive assay techniques, such as mass spectrometry and immunoassays, enable precise identification of blood biomarkers present in minimal concentrations. Researchers use growing artificial intelligence and machine learning combinations to enhance diagnostic precision, along with data analysis capabilities. Various governments implement funding initiatives and regulatory measures to accelerate diagnostic tool development and market growth through their support of Alzheimer's research.
Type Insights
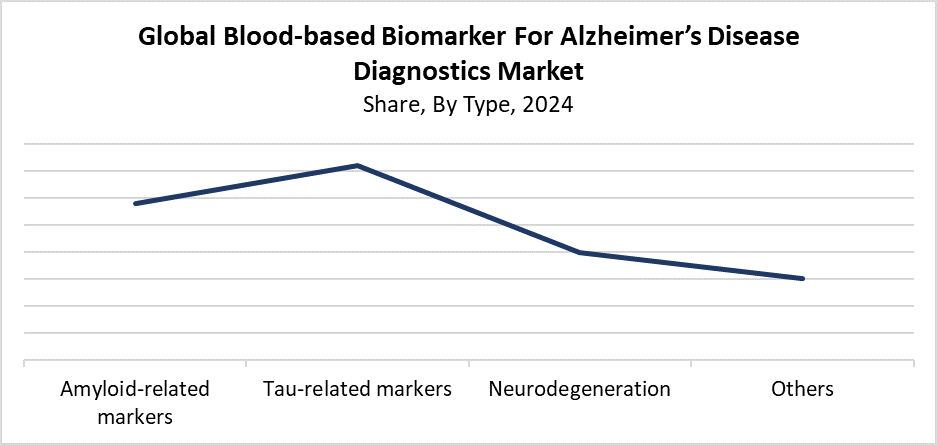
The tau-related markers segment dominated the blood-based biomarker for Alzheimer's disease diagnostics market with the largest revenue share of 36.4% in 2024. The high market dominance stems from tau proteins, which show strong connections with Alzheimer's disease progression, making them valuable markers for detecting the disease in its early stages. Blood levels of phosphorylated tau (p-tau) serve as specific indicators of neurodegeneration, which researchers now broadly accept to distinguish Alzheimer's from other dementia types. The detection of tau markers has become more precise through technological improvements in assay technology, which strengthens their clinical implementation. Researchers expect this market to maintain its leadership position because precise non-invasive diagnostic methods remain in demand as long as tau diagnostics receive proper validation in studies.
The amyloid-related markers segment in the blood-based biomarker for Alzheimer’s disease diagnostics market is predicted to experience the fastest growth during the projection timeframe. The rising evidence about amyloid-beta accumulation as a primary early change in Alzheimer's disease stands as the main factor driving this market growth. The blood-based methods that track amyloid-beta levels present more convenience and less invasiveness than both PET scans and CSF analyses. Technological developments have significantly boosted plasma amyloid-beta detection sensitivity, which enables enhanced early diagnosis capabilities for mass population screening. The segment will experience fast growth due to the rising demand for precise amyloid biomarkers, which follows the increasing number of clinical trials and medication investigations focused on amyloid pathology.
Technology Insights
The immunoassay segment held the largest market share of 52.62% in 2024 and led the blood-based biomarker for Alzheimer's disease diagnosis market. The main reason behind this dominance stems from the widespread application of immunoassay approaches, which detect proteins including tau and amyloid-beta with high precision and sensitivity. Immunoassays, including ELISA and SIMOA, deliver testing solutions that scale easily and cost-effectively and work for both clinical and research settings. Early diagnostic capabilities improved substantially because these assays can detect blood biomarkers at very low concentrations. The established status of immunoassays as the standard diagnostic method for Alzheimer's biomarkers continues to grow because of continuous improvements in assay design alongside automation developments.
The next-generation platforms segment of the blood-based biomarker for Alzheimer's disease diagnostics market is anticipated to grow at the fastest CAGR during the forecast period. The rapid expansion of these systems stems from technological advances, including digital PCR, multiplex assays, and mass spectrometry, which deliver enhanced sensitivity and accuracy together with multiplexing capabilities beyond traditional methods. The systems enable simultaneous detection of multiple biomarkers by analyzing minimal blood samples, thus allowing precise diagnostics. Machine learning and artificial intelligence systems enhance data analysis and predictive capabilities through their integration. The rising demand for precise early diagnosis tools that do not require invasive procedures has propelled next-generation platforms to become fundamental drivers of innovation and future market growth.
Regional Insights
In 2024, North America led the blood-based biomarker market for Alzheimer’s disease diagnostics with the largest revenue share of 46.53%. The region maintains market leadership because it adopted modern diagnostic technologies early, as well as due to its extensive Alzheimer’s research funding and well-developed healthcare systems. Blood-based biomarker test development, along with marketing processes, has been accelerated because major market players exist alongside numerous ongoing clinical trials. The industry benefits from excellent growth potential since government entities, including the FDA, provide regulatory backing, together with financial assistance. North America maintains its dominant position in the worldwide market because its population ages, while public understanding of Alzheimer’s early diagnosis grows.
Europe Blood-based Biomarker for Alzheimer’s Disease Diagnostics Market Trends
The blood-based biomarker for Alzheimer's disease diagnostics market is rapidly expanding throughout Europe because of rising healthcare awareness and a growing number of Alzheimer's disease cases. The adoption of cutting-edge diagnostic technologies is facilitated by Europe's well-established healthcare systems and strong emphasis on early disease identification. Developments in biomarker discovery and clinical validation are being propelled by government programs and financing initiatives focused on neurodegenerative disease research. Innovation and market penetration are being accelerated through healthcare provider collaboration with biotech firms and academic institutions. European regulatory bodies speed up the approval process for new diagnostic technology to boost market expansion. The blood-based biomarker market in Europe will experience steady growth during the forecast period because of an aging population coupled with increasing demand for affordable non-invasive diagnostic options.
Asia Pacific Blood-based Biomarker for Alzheimer’s Disease Diagnostics Market Trends
The Asia-Pacific market for blood-based biomarkers to diagnose Alzheimer's disease shows significant growth because of fast population aging and rising regional recognition of this disease. Modern diagnostic tools have become more accessible to Chinese, Japanese, and Indian populations thanks to increased healthcare investments and improved healthcare systems. Government funding, along with national health programs, drives faster research development of neurodegenerative diseases. Blood-based biomarker tests serve the growing market need for diagnostic options that are both affordable and non-invasive and enable early detection. Regional biotech companies in Asia-Pacific are building new partnerships with international enterprises, which propels technological advancement and makes the region a key developing market with strong growth potential in future years.
Key Blood-based Biomarker For Alzheimer’s Disease Diagnostics Companies:
The following are the leading companies in the blood-based biomarker for Alzheimer’s disease diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers
- Quest Diagnostics Incorporated
- Quanterix
- Fujirebio
- Lilly USA, LLC
- Labcorp
- Abbott
- C2N Diagnostics
- ALZpath
- Others
Recent Developments
- In July 2025, the Alzheimer's Association released its first clinical practice guideline (CPG) for the diagnosis, management, and care of Alzheimer's disease and related dementias during the Alzheimer's Association International Conference (AAIC) in Toronto. The use of blood-based biomarker (BBM) tests by experts to assess Alzheimer's pathology in patients with cognitive impairment is emphasized in this first recommendation. The rule offers evidence-based resources to help with earlier and more accurate diagnosis, guaranteeing prompt access to appropriate treatment. It was created as part of the Association's ALZPro program, a centralized hub for dementia specialists.
- In May 2025, Fujirebio declared that its Lumipulse G pTau 217/β-Amyloid 1-42 Plasma Ratio in-vitro diagnostic (IVD) test has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The test was created to measure amyloid pathology in patients being assessed for Alzheimer's disease or other causes of cognitive loss, and the FDA has previously designated it as a Breakthrough Device. It now becomes the first FDA-cleared blood-based IVD test in the U.S. to support the identification of patients with amyloid pathology linked to Alzheimer’s disease (AD).
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the blood-based biomarker for alzheimer’s disease diagnostics market based on the below-mentioned segments:
Global Blood-based Biomarker For Alzheimer’s Disease Diagnostics Market, By Type
- Amyloid-related markers
- Tau-related markers
- Neurodegeneration
- Others
Global Blood-based Biomarker For Alzheimer’s Disease Diagnostics Market, By Technology
- Immunoassays
- Mass spectrometry-based assays
- Next-generation platforms
- Others
Global Blood-based Biomarker For Alzheimer’s Disease Diagnostics Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Global |
| Pages | 233 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Sep 2025 |
| Access | Download from this page |
