Global Blood-based Biomarker For Parkinsons Disease Market
Global Blood-based Biomarker For Parkinsons Disease Market Size, Share, and COVID-19 Impact Analysis, By Biomarker (Protein Biomarkers, Inflammatory Markers, Metabolic markers, Transcriptomic/miRNA, Others), By Technology (ELISA/Immunoassay, Multiplex platforms, NGS/qPCR (genetic), Mass spectrometry, Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025-2035.
Report Overview
Table of Contents
Blood-based Biomarker For Parkinson’s Disease Market Summary
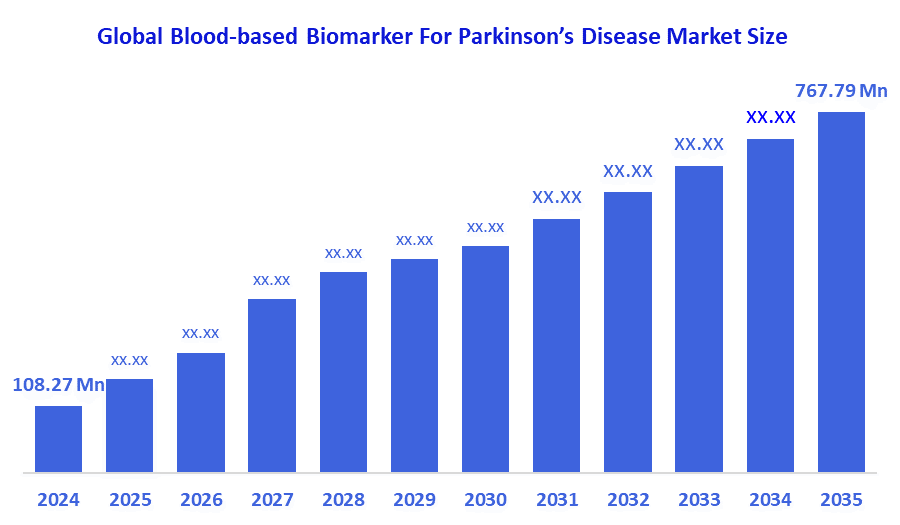
The Global Blood-Based Biomarker For Parkinson’s Disease Market Size Was Estimated at USD 108.27 Million in 2024 and is Projected to Reach USD 767.79 Million by 2035, Growing at a CAGR of 19.49% from 2025 to 2035. The market for blood-based biomarkers for Parkinson's disease is expanding due to factors such as increased disease prevalence, the need for early detection, non-invasive testing techniques, and developments in biomarker validation and discovery.
Key Regional and Segment-Wise Insights
- In 2024, North America accounted for 42.76% of the global market for blood-based biomarkers for Parkinson's disease.
- In 2024, the protein biomarkers segment had the biggest market share of 54.42% based on biomarkers.
- In 2024, the ELISA/Immunoassay segment had the largest market share of 43.62% based on technology.
Global Market Forecast and Revenue Outlook
- 2024 Market Size: USD 108.27 Million
- 2035 Projected Market Size: USD 767.79 Million
- CAGR (2025-2035): 19.49%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Blood-based biomarkers for Parkinson's disease represent a market that focuses on developing diagnostic tools that detect biological markers within blood samples. Blood-based biomarkers serve as a substitute to traditional diagnostic methods because they offer non-invasive, affordable accessibility and can detect diseases at early stages, which imaging and clinical assessments typically fail to identify. The Parkinson's disease market continues to expand due to three key factors: better diagnostic methods, worldwide disease growth, and increased patient and healthcare professional understanding. The rising interest in personalized medicine alongside early intervention methods drives the growing requirement for dependable blood-based diagnostic solutions that enable timely treatment choices and improved patient results.
The market for blood-based biomarkers experiences a major transformation through technological breakthroughs. Researchers now have better means to discover and prove new Parkinson's disease biomarkers because of recent developments in proteomics alongside genomics, and high-throughput screening techniques. Research and development efforts in this area are also being accelerated by rising public and private sector investments. National health policies and research funding for neurodegenerative diseases by government programs promote progress. The combined efforts of these initiatives will drive discoveries while improving diagnostic accuracy and expanding blood-based biomarker applications for Parkinson's disease treatment.
Biomarker Insights
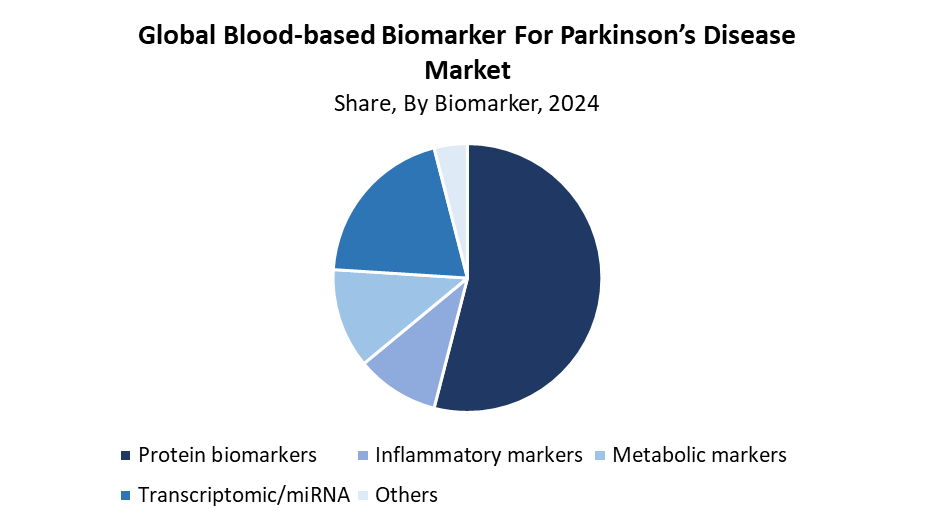
What Factors Enabled the Protein Biomarker Segment to Capture a 54.42% Revenue Share in the Blood-Based Biomarker for Parkinson’s Disease Market in 2024?
The protein biomarker segment dominated the blood-based biomarker for Parkinson’s disease market by capturing the largest revenue share of 54.42% in 2024. The pathophysiological importance of specific proteins, including alpha-synuclein, tau, and neurofilament light chain, explains their market leadership. Scientists examine these protein biomarkers extensively to detect Parkinson’s disease early and track disease progression as well as evaluate treatment effectiveness. Modern proteomic technologies have expanded blood sample biomarker detection, which makes protein biomarkers the most commonly used markers in diagnostics and clinical research. The established connection between these biomarkers and neurodegenerative conditions has driven their market dominance in 2024.
The transcriptomic/miRNA segment of the blood-based biomarker for Parkinson's disease market is anticipated to grow at the fastest CAGR during the projection period. The rapid increase of transcriptomic markers, including miRNAs, stems from their emerging role as crucial gene expression regulators that link to neurodegenerative disease development and progression. The biomarkers enable painless blood testing, which delivers precise results and high sensitivity that helps identify diseases early and track their progression. The rate of discovery and validation for new transcriptome biomarkers has accelerated because of progress in bioinformatics tools, together with RNA sequencing technologies. The advancement in this field receives additional support through increasing research funding, as well as pharmaceutical companies and biotech firms forming partnerships with academic institutions.
Technology Insights
How Did the ELISA/Immunoassay Segment Lead the Blood-Based Biomarkers for Parkinson’s Disease Market in 2024?
The ELISA/Immunoassay segment led the blood-based biomarkers for Parkinson's disease market by generating the largest revenue share of 43.62% in 2024. Immunoassay and ELISA (enzyme-linked immunosorbent assay) techniques dominate the market because they provide precise, affordable protein biomarker detection and maintain high sensitivity and specificity for Parkinson's disease diagnosis. These assays serve as standard tools for both clinical and research applications to detect critical blood proteins, including tau and alpha-synuclein. The assays gain popularity for regular diagnostics and biomarker validation because they scale well while being easy to use and compatible with automated screening systems. The market leads continue to be dominated by ELISA/Immunoassay technologies.
The multiplex platforms segment of the blood-based biomarkers for Parkinson's disease market is anticipated to grow at the fastest CAGR during the forecast period. High-throughput diagnostic instruments that analyze multiple biomarkers from one sample simultaneously drive the expansion of multiplex platforms. The multiplex platforms show superior efficiency and precision, together with cost-effectiveness, compared to traditional single-analyte methods. Researchers and clinicians use these technologies increasingly to detect complex biomarker patterns that show how Parkinson's disease progresses and how treatments work. The efficiency of multiplex systems improves through the development of microarrays and microfluidics, and bead-based assays. Increased financial support for precision medicine, along with neurodegenerative disease research, has led to greater adoption of multiplex diagnostic technologies.
Regional Insights
North America held the largest revenue share of 42.76% and led the market of blood-based biomarkers for Parkinson's disease globally. The region stands out because it hosts numerous major market players alongside advanced healthcare systems and substantial funding for neurodegenerative disease research. The market experienced growth due to increasing patient need for early non-invasive diagnostic procedures combined with the expanding number of Parkinson's disease cases. The research for biomarkers received a boost from government programs and financial support, which encouraged innovation and accelerated new product development. The United States stood out because it had both a high number of clinical trials and programs to educate patients about medical conditions. The combination of these factors led North America to establish the highest position in the 2024 global market for Parkinson's blood-based biomarker diagnostics.
Europe Blood-Based Biomarker For Parkinson’s Disease Market Trends
The European blood-based biomarker for Parkinson's disease market is experiencing rapid expansion because of rising disease prevalence and the demand for early detection methods through blood tests. The development of sensitive blood-based biomarkers advanced at a rapid pace because of both enhanced disease pathology understanding and biotechnology improvements, which enabled earlier patient detection and better disease tracking. Neurodegenerative research development keeps growing because governments now support it with new funding and financial resources. The medical community and patients increasingly choose blood-based diagnostics after understanding their benefits. Major industry leaders dedicate substantial funding to scientific investigations along with strategic alliances to enhance biomarker availability and diagnostic precision. The expanding senior population alongside personalized medicine developments drives the healthcare market expansion, which establishes Europe as the frontrunner in this emerging medical field.
Asia Pacific Blood-based Biomarker For Parkinson’s Disease Market Trends
The blood-based biomarkers for Parkinson's disease market in the Asia-Pacific is anticipated to grow at the fastest CAGR throughout the forecasted period. Urbanization, along with aging populations, has led to a rapidly growing number of Parkinson's disease cases, which creates a rising demand for early diagnostic solutions. Biotechnology advancements, along with healthcare infrastructure development in the region, are accelerating the adoption of blood-based biomarkers. The industry experiences growth from increased government funding alongside initiatives that focus on neurodegenerative disease research. The market growth receives additional support through rising awareness among patients and physicians about testing methods that are less invasive. The development of blood-based Parkinson's disease biomarkers in Asia-Pacific receives momentum from regional and international collaborations, which establish the region as a fast-growing research hub.
Key Blood-Based Biomarkers For Parkinson’s Disease Companies:
The following are the leading companies in the blood-based biomarker for Parkinson’s disease market. These companies collectively hold the largest market share and dictate industry trends.
- Abbott
- Rules-Based Medicine
- ACOBIOM
- Banyan Biomarkers
- QIAGEN
- Quanterix
- Bio-Rad Laboratories
- Johnson & Johnson Services
- Merck KGaA
- Alseres Pharmaceuticals
- DiaGenic ASA
- Thermo Fisher Scientific
- Others
Recent Developments
- In May 2025, Rune Labs and the Parkinson’s Foundation launched the first clinical initiative aimed at linking genetic biomarkers with digital health data to enhance understanding and management of Parkinson’s disease.
- In April 2025, using blood-based biomarkers, FYR and the Mayo Clinic teamed up to further Parkinson's research with the goal of better understanding disease progression and subtypes to enhance patient treatment and clinical trial success.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the blood-based biomarker for parkinson’s disease market based on the below-mentioned segments:
Global Blood-based Biomarker For Parkinson’s Disease Market, By Biomarker
- Protein biomarkers
- Inflammatory markers
- Metabolic markers
- Transcriptomic/miRNA
- Others
Global Blood-based Biomarker For Parkinson’s Disease Market, By Technology
- ELISA/Immunoassay
- Multiplex platforms
- NGS/qPCR (genetic)
- Mass spectrometry
- Others
Global Blood-based Biomarker For Parkinson’s Disease Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Global |
| Pages | 240 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Sep 2025 |
| Access | Download from this page |
