Brazil Biological Safety Testing Market
Brazil Biological Safety Testing Market Size, Share, and COVID-19 Impact Analysis, By Services (Biological Testing Service, Endotoxin Testing Service, Sterility Testing Service, Viral Clearence and Validation Service, Cell Line Authentication and Characterization Test, Residual Host Contamination Testing Services, Adventitious Agent Detection Testing Services, and Others), By Application (Vaccine & Therapeutics, Monoclonal Antibiotics, Vaccines, Recombinant Protein, Cell and Gene Therapy, Blood and Blood Products, Tissue and Tissue- based Product, and Others), and Brazil Biological Safety Testing Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Brazil Biological Safety Testing Market Size Insights Forecasts to 2035
- The Brazil Biological Safety Testing Market Size was estimated at USD 157.9 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 10.33 % from 2025 to 2035
- The Brazil Biological Safety Testing Market Size is Expected to Reach USD 465.5 Million by 2035
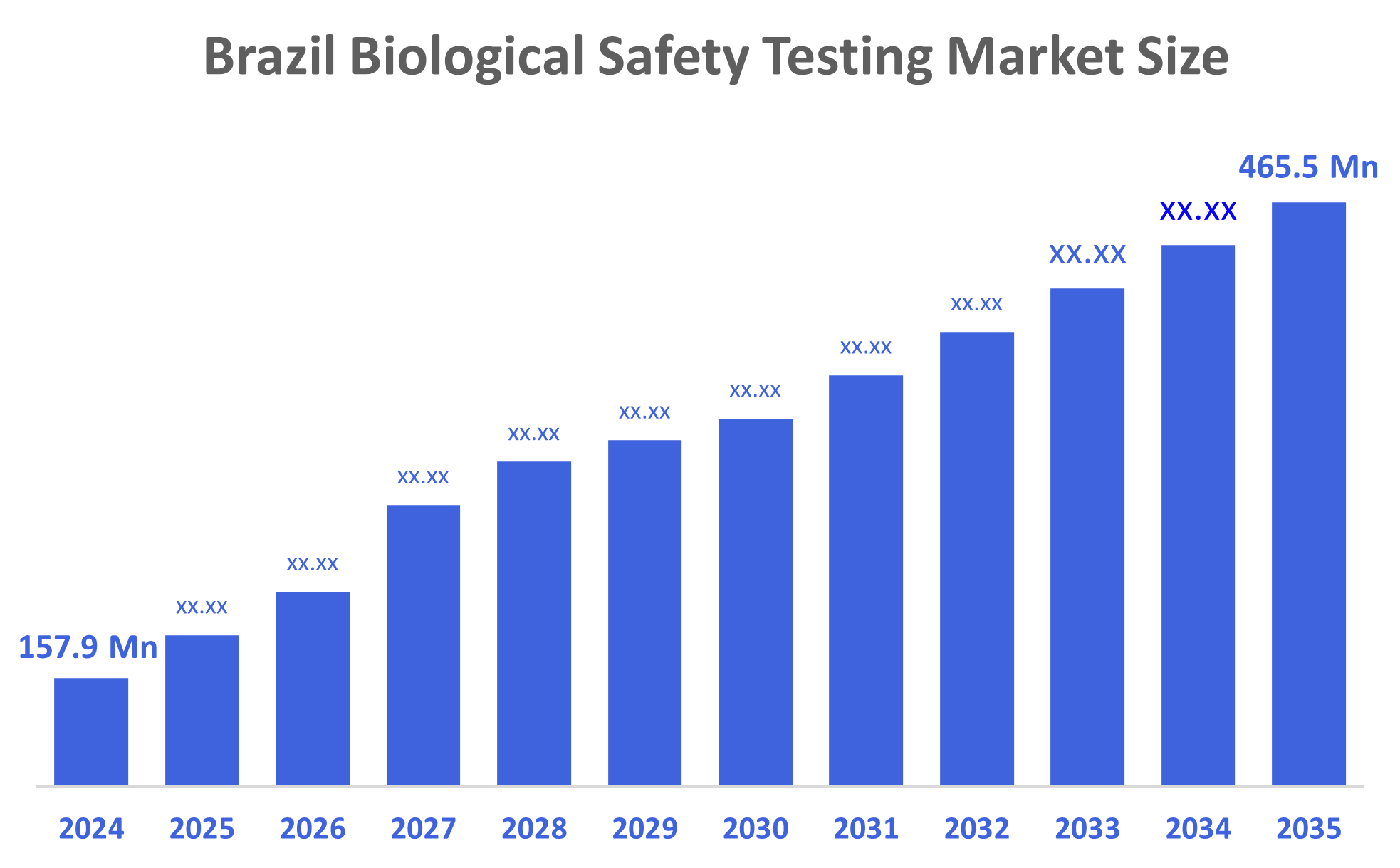
According to a research report published by Decisions Advisors, The Brazil Biological Safety Testing Market Size is Anticipated to Reach USD 465.5 Million by 2035, Growing at a CAGR of 10.33 % from 2025 to 2035. The Brazil biological safety testing market is driven by increasing rates of Infectious Diseases and the increasing number of Contract Research Organisations and Manufacturing Organisations are other aspects of growth factors for Brazil. Additionally, increased global agreements, the use of automated and digital testing systems, and the requirement for accreditation for exports, as well as the introduction of cellular and gene therapy are some of the more significant drivers of growth in Brazil.
Market Overview
Biological safety testing is the application of principles, technologies, and practices designed to prevent unintended exposure of people and the environment to potentially infectious agents. Additionally, the market has many opportunities available to it because of an expanding pharmaceutical and biopharmaceutical sector, increasing production of vaccines and biosimilars, more stringent regulatory compliance regulations, an influx of Clinical Trials into the country, increased manufacturing activity in biologics, and increased government support for both health care innovation and quality assurance infrastructure. Additionally, the Brazilian government promotes the biological safety testing field by creating comprehensive regulations through ANVISA, increasing financial support for vaccine and public health initiatives, creating incentives for domestic biopharmaceutical production, providing support for clinical studies, and investing in laboratory facilities, biosecurity criteria, and quality assurance systems.
Report Coverage
This research report categorizes the market for the Brazil biological safety testing market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Brazil biological safety testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Brazil biological safety testing market.
Driving Factors
The Brazil biological safety testing market is driven by increased number of vaccine, biologics and biosimilars produced, the increased regulations from ANVISA to ensure a quality and safe product, the increased pharmaceutical and biotechnology R&D activity, the increased number of clinical trials conducted in Brazil, increased public immunisation programs, increased levels of awareness of biosafety standards, outsourcing of testing services and continuing investment into lab infrastructure and advanced analytical technologies.
Restraining Factors
The Brazil biological safety testing market is restrained by the limitations that are hindering growth. The major limiting factors are the high costs for testing, compliance with regulations, and lengthy processes required to obtain regulatory approval. Additionally, limitations include a lack of available-trained professionals and high initial capital expenditures needed to establish advanced labs, as well as extended turnaround times needed to validate and certify biological safety test results.
Market Segmentation
The Brazil biological safety testing market share is classified into services and applications.
- The sterility testing service segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Brazil biological safety testing market is segmented by services into biological testing service, endotoxin testing service, sterility testing service, viral clearance and validation service, cell line authentication and characterization test, residual host contamination testing services, adventitious agent detection testing services, and others. Among these, the sterility testing service segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental growth is due required levels of sterility for vaccines, biologics, injectable drugs, and medical devices, increased domestic production of vaccines and clinical trials, stricter ANVISA regulations, and the increasing demand for contract services provided by certified sterile testing laboratories within Brazil.
- The vaccine & therapeutics segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
The Brazil biological safety testing market is segmented by applications into vaccine & therapeutics, monoclonal antibiotics, vaccines, recombinant protein, cell and gene therapy, blood and blood products, tissue, and tissue- based product, and others. Among these, the vaccine & therapeutics segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The growth of the segment is due to large public immunization programs, an expanding production of biologics and biosimilar products, stringent ANVISA safety requirements, frequent testing of each production batch, increasing amounts of clinical trials, and the government's continued investment into vaccine and therapeutic manufacturing capacity.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Brazil biological safety testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Charles River Laboratories International
- Merck KGaA
- Lonza Group Ltd.
- Eurofins Scientific
- SGS SA
- WuXi AppTec
- Toxikon Corporation
- Avista Pharma Solutions
- Nelson Laboratories
- Pace Analytical Services
- Other
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Brazil, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the Brazil Biological Safety Testing Market based on the below-mentioned segments:
Brazil Biological Safety Testing Market, By Service
- Biological Testing service
- Endotoxin Testing service
- Sterility Testing service
- Viral Clearence and Validation Service
- Cell Line Authentication and Characterization Test
- Residual Host Contamination Testing Services
- Adventitious Agent Detection Testing Services
- Others
Brazil Biological Safety Testing Market, By Application
- Vaccine & Therapeutics
- Monoclonal Antibiotics
- Vaccines
- Recombinant Protein
- Cell and Gene Therapy
- Blood and Blood Products
- Tissue and Tissue- based Product
- Others
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 225 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Dec 2025 |
| Access | Download from this page |
