Brazil Clinical Trials Market
Brazil Clinical Trial Market Size, Share, and COVID-19 Impact Analysis, By Phase (Phase I, Phase II, Phase III, and Phase IV), By Application (Oncology, CNS Disorder, Cardiology, Infectious Disease, Metabolic Disorder, Renal/Nephrology, and Others), and Brazil Clinical Trial Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Brazil Clinical Trial Market Size Insights Forecasts to 2035
- The Brazil Clinical Trial Market Size Was Estimated at USD 420.60 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 6.5% from 2025 to 2035
- The Brazil Clinical Trial Market Size is Expected to Reach USD 840 Million by 2035
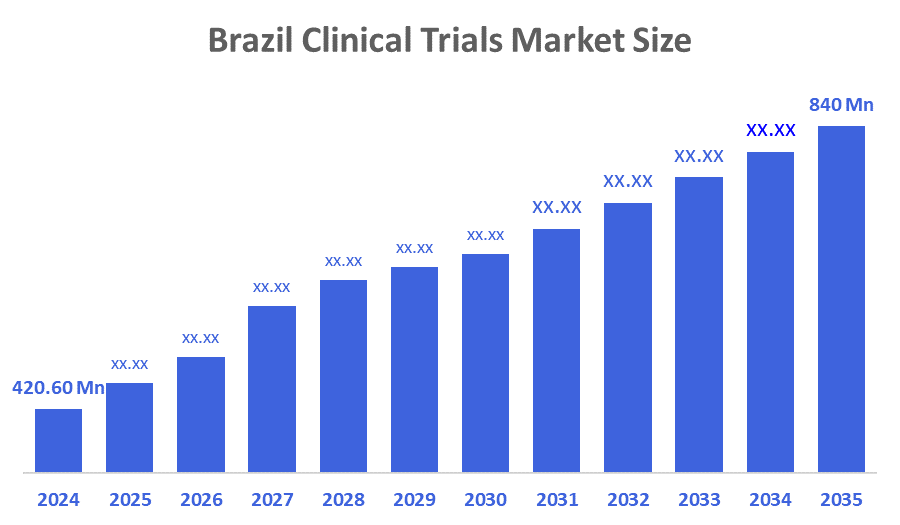
According to a Research Report Published by Decisions Advisors & Consulting, The Brazil Clinical Trial Market Size is anticipated to Reach USD 840 Million by 2035, Growing at a CAGR of 6.5% from 2025 to 2035. Clinical trials are the processes that aid in researching the safety and efficacy of new treatments, therapeutics, and medical devices. The increasing prevalence of chronic diseases has increased the demand for effective diagnostics and therapeutics. This factor has been increasing the number of trials conducted for the development of effective therapeutics, thus contributing to the market growth.
Market Overview
A clinical trial is a systematic study conducted on human subjects to evaluate the safety, efficacy, and quality of new drugs, medical devices, or therapies. The consumers volunteer to participate in clinical trials to test medical interventions, including drugs, cells, and other biological products, surgical procedures, radiological procedures, devices, and preventive care. Clinical trials are carefully designed, reviewed, and completed, and need to be approved before they can start. Individuals of all ages can take part in clinical trials, including children. The clinical trial can be divided into five phases, with every phase playing a distinct purpose within the clinical trial. Every trial adheres to a procedure that designates what types of individuals may participate in the study. According to the Regulatory Focus factsheet, Brazil registered around 10,000 clinical studies by April 2024. Clinical trials are essential for advancing medical knowledge and ensuring the approval of new therapies. The market is growing rapidly due to favourable government policies, increasing investments, and a large, diverse patient population. Brazil's expanding healthcare infrastructure, combined with a rising demand for clinical research activities, makes the country an attractive destination for conducting clinical trials.
Report Coverage
This research report categorizes the market for the Brazil clinical trial market based on various segments and regions, and forecasts revenue growth and analysis trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Brazil clinical trial market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Brazil clinical trial market.
Driving Factors
The Brazil clinical trial market is driven by factors such as its genetically diverse population ideal for global studies, rising collaborations between universities and CROs, supportive government incentives for R&D, cost-effective trial operations, advanced digital health adoption, and increasing participation of local biotech startups focusing on innovative drug discovery and precision medicine research. Additionally, Brazil’s growing regulatory efficiency, expanding clinical research infrastructure, availability of skilled medical professionals, rising interest from international pharmaceutical companies, and increased public-private partnerships further strengthen the nation’s position as a leading hub for high-quality, multicenter clinical trials in Latin America.
Restraining Factors
The Brazil clinical trial market faces restraints such as complex regulatory procedures, limited funding for early-stage research, uneven infrastructure across regions, participant recruitment challenges, ethical approval delays, and data privacy concerns impacting trial efficiency and international collaboration.
Market Segmentation
The Brazil clinical trial market share is categorized by phase and application.
The phase III segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Brazil clinical trial market is segmented by phase into phase I, phase II, phase III, and phase IV. Among these, the phase III segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental growth due to the increasing number of studies being registered in the country. This growth is attributed to factors like the high prevalence of diseases such as cancer, which drives the need for new therapies and trials, and an increase in the number of phase III studies being conducted.
The oncology segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Brazil clinical trial market is segmented by application into oncology, CNS disorder, cardiology, infectious disease, metabolic disorder, renal/nephrology, and others. Among these, the oncology segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The market is driven by the rising incidence of cancer, growing investments in oncology research, availability of advanced diagnostic technologies, and strong collaborations between global pharmaceutical companies and local research centers, leading to accelerated drug development, personalized treatment approaches, and increased patient participation in cancer-focused clinical trials.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Brazil clinical trial market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- IQVIA Inc. (U.S.)
- ICON plc (Ireland)
- Parexel (U.S.)
- Charles River Laboratories (U.S.)
- Laboratory Corporation of America Holdings (LabCorp/Covance) (U.S.)
- Medpace Holdings, Inc. (U.S.)
- PPD (Thermo Fisher Scientific Inc.) (U.S.)
- Syneos Health (U.S.)
- PRA Health Sciences (U.S.)
- Other
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In January 2025: The ICH Guidelines for Good Clinical Practice E6(R3), which Brazil has adopted, were finalized.
Market Segment
This study forecasts revenue at the Brazil, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the Brazilian clinical trial Market based on the following segments:
Brazil Clinical Trial Market, By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Brazil Clinical Trial Market, By Application
- Oncology
- CNS Disorder
- Cardiology
- Infectious Disease
- Metabolic Disorder
- Renal/Nephrology
- Others
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 258 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
