Brazil In Vitro Diagnostics Market
Brazil In Vitro Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Clinical Chemistry, Molecular Diagnostics, Immunodiagnostics, Hematology, Other), by Product (Instrument, Reagents, Other), and Brazil In Vitro Diagnostics Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Brazil In Vitro Diagnostics Market Insights Forecasts to 2035
- The Brazil In Vitro Diagnostics Market Size Was Estimated at USD 1.8 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of Around 5.8% from 2025 to 2035
- The Brazil In Vitro Diagnostics Market Size is Expected to Reach USD 3.35 Billion by 2035
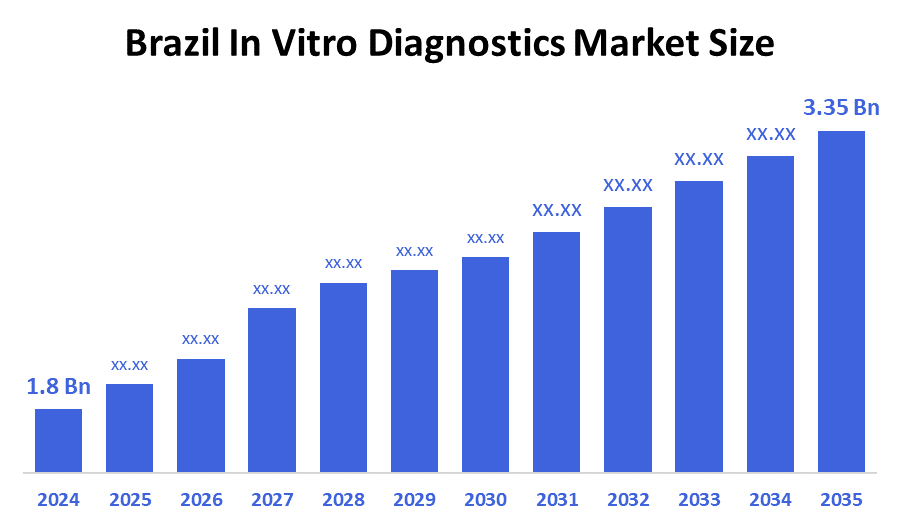
According to a research report published by Decision advisor & Consulting, the Brazil In Vitro Diagnostics Market size is anticipated to reach USD 3.35 billion by 2035, growing at a CAGR of 5.8% from 2025 to 2035. Brazil’s in vitro diagnostics market is growing due to rising chronic diseases, increasing demand for early and accurate diagnosis, expanding healthcare access, and government investments in laboratory infrastructure. Technological advancements, rapid tests, and greater awareness of preventive healthcare also support market growth, especially in urban and semi-urban areas.
Market Overview
In vitro diagnostics are medical tests done on samples like blood, urine, or tissue taken from the body. These tests help doctors detect diseases, check health conditions, and monitor treatment without directly entering the body. They are used in labs, hospitals, and even home test kits to give quick, reliable health information. Additionally, Brazil in vitro diagnostic market is growing due to rising chronic diseases, demand for early diagnosis, and expanding laboratory services. Government policies supporting universal healthcare (SUS), improved reimbursement, and investments in diagnostic infrastructure also boost adoption. Increased use of rapid tests and advanced technologies further drives strong market growth across the country. Additionally, New technology in the in vitro diagnostics (IVD) market includes rapid point-of-care tests, advanced molecular diagnostics (like PCR and genetic testing), AI-based diagnostic tools, digital pathology, and fully automated analysers. These innovations make testing faster, more accurate, and easier to access, helping doctors detect diseases early and improving overall patient care. For instance, as per a May 2020 update, Brazil's National Health Surveillance Agency (ANVISA) expedited emergency reviews and approvals for medical devices and in-vitro diagnostics (IVDs) related to the COVID-19 pandemic
Report Coverage
This research report categorizes the market for the Brazil in vitro diagnostics market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Brazil in vitro diagnostics market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Brazil in vitro diagnostics market.
Driving Factors
Brazil’s In Vitro Diagnostics market is driven by rising chronic and infectious diseases, increasing use of molecular diagnostics like PCR and genetic testing, and the rapid adoption of point-of-care technologies. Government support through SUS investments, improved regulations by ANVISA, and encouragement of local manufacturing further boost growth. Recent developments such as AI-based diagnostic tools, digital pathology, automated analyzers, and home-testing kits are expanding access and improving accuracy. Growing awareness of early diagnosis and better healthcare infrastructure also contributes to the strong market expansion across the country.
Restraining Factors
Brazil In Vitro Diagnostics market faces restraints such as high costs of advanced diagnostic equipment, limited access to modern labs in rural regions, and dependence on imported reagents, which can cause supply delays. Complex regulatory processes, shortage of skilled laboratory professionals, and budget constraints in public healthcare also slow the adoption of new technologies across the country.
Market Segmentation
The Brazil in vitro diagnostics market share is categorized by test type and product.
- The immunodiagnostics segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Brazil in vitro diagnostics market is segmented by test type into clinical chemistry, molecular diagnostics, immunodiagnostics, hematology, and other. Among these, the immunodiagnostics segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental growth is driven by it is essential for diagnosing a wide range of common diseases, including infections, hormonal disorders, autoimmune conditions, and chronic illnesses. These tests are highly reliable, easy to automate, and suitable for high-volume testing in hospitals and laboratories. Brazil frequently deals with infectious outbreaks, increasing the need for rapid and accurate immunoassay testing. Technological advancements such as automated analyzers and chemiluminescence systems have further improved speed and accuracy. Additionally, immunodiagnostic tests are widely used in routine health check-ups, making them one of the most demanded and trusted diagnostic methods across the country.
- The reagents segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
The Brazil in vitro diagnostics market is segmented by product into instruments, reagents, and other. Among these, the reagent segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. The growth of the segment is due to they are the core materials needed for every test performed in labs, hospitals, and point-of-care settings. While instruments are purchased occasionally, reagents are used daily and must be continuously replenished, creating steady and high demand. Their use spans all major test types such as immunoassays, molecular diagnostics, hematology, and clinical chemistry. Growing testing volumes for chronic and infectious diseases further increase reagent consumption. Advances in specialized kits, automation-compatible reagents, and high-sensitivity formulations also boost their importance, making reagents the largest and most consistently used product segment in the country.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Brazil in vitro diagnostics market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Roche Diagnostics
- Abbott Laboratories
- Danaher Corporation (Beckman Coulter)
- Siemens Healthineers
- Thermo Fisher Scientific
- Becton, Dickinson & Company (BD)
- bioMérieux
- Sysmex Corporation
- Bio-Rad Laboratories
- Other
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent News
In November 2020, Chembio Diagnostics, Inc., a company that makes quick testing kits for infectious diseases, announced that its Brazil branch received approval from ANVISA (Brazil’s health authority) for its DPP SARS-CoV-2 Antigen test. This approval allowed the company to sell and use the COVID-19 rapid test in Brazil.
Market Segment
This study forecasts revenue at the Brazil, regional, and country levels from 2020 to 2035. decision advisor has segmented the Brazil in vitro diagnostics market based on the below-mentioned segments:
Brazil In Vitro Diagnostics Market, By Test Type
- Clinical Chemistry
- Molecular Diagnostics
- Immunodiagnostics
- Hematology
- Other
Brazil In Vitro Diagnostics Market, By Product
- Instrument
- Reagents
- Other
FAQ’s
1. What is in vitro diagnostics (IVD)?
- IVD refers to medical tests performed on samples like blood, urine, or tissue to detect diseases, monitor health, or guide treatment.
2. What drives the growth of Brazil’s IVD market?
- Rising chronic diseases, demand for early diagnosis, new technologies, rapid tests, and government support for healthcare expansion.
3. Which test type dominates the Brazil IVD market?
- Immunodiagnostics dominates due to their wide use in detecting infections and chronic diseases.
4. Which product segment is the largest?
- Reagents lead because they are required for every diagnostic test and are used repeatedly.
5. What new technologies are growing in the market?
- Molecular diagnostics, PCR, digital pathology, AI-based tools, automated analyzers, and point-of-care tests.
6. What are the key restraining factors?
- High equipment costs, limited lab access in rural areas, skilled worker shortages, and dependence on imported reagents.
7. Who regulates IVD products in Brazil?
- ANVISA (Agência Nacional de Vigilância Sanitária) regulates and approves diagnostic products and technologies.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | country |
| Pages | 184 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
