Canada Medical Writing Market
Canada Medical Writing Market Size, Share, and COVID-19 Impact Analysis, By Type (Clinical Writing, Regulatory Writing, Scientific Writing, and Others), By Application (Medical Journalism, Medical Education, Medico Marketing, and Others), and Canada Medical Writing Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
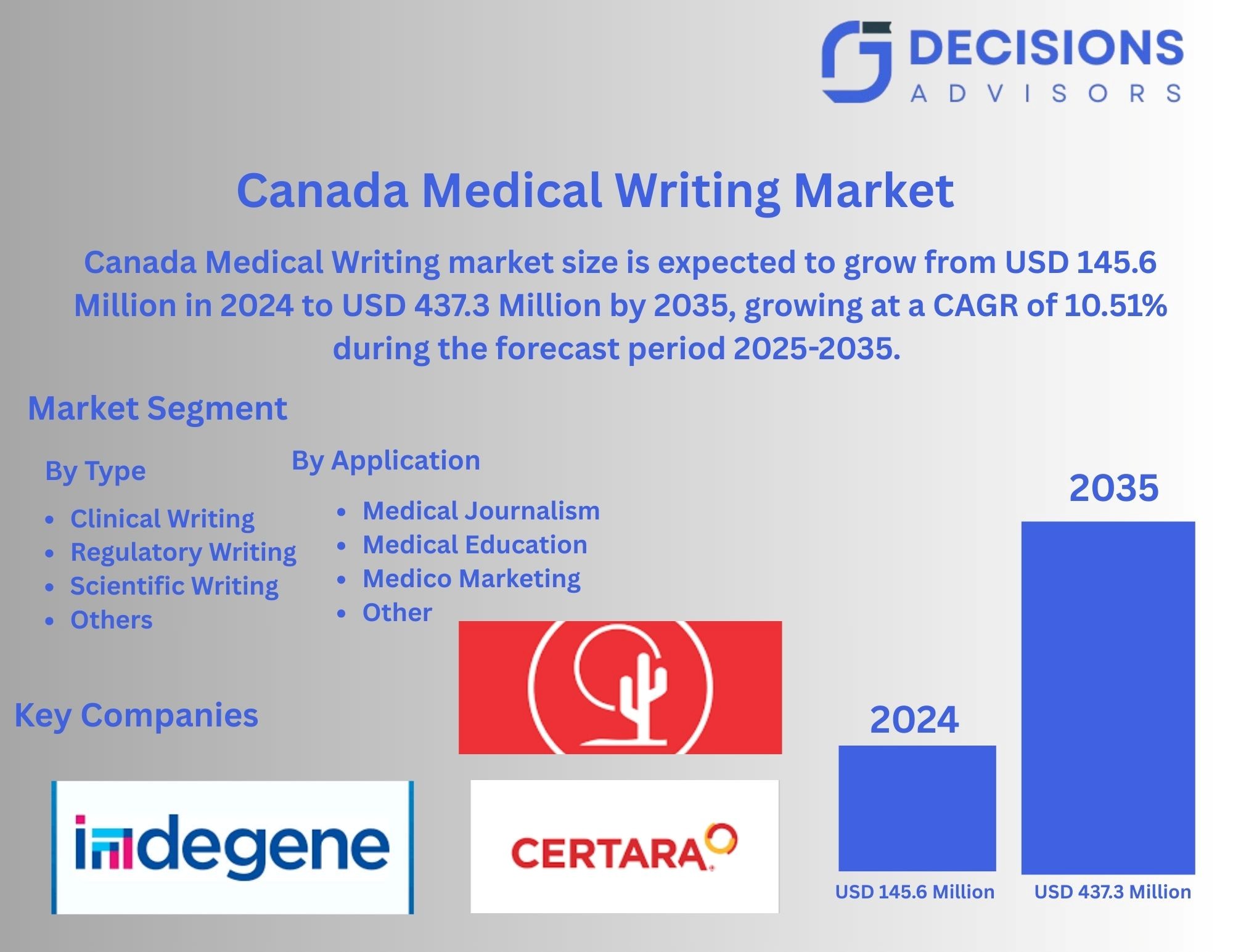
Canada Medical Writing Market Insights Forecasts to 2035
- The Canada Medical Writing Market Was Estimated at USD 145.6 Million in 2024.
- The Market Size is Growing at a CAGR of 10.51% between 2025 and 2035.
- The Canada Medical Writing market is Anticipated to Reach USD 437.3 Million by 2035.
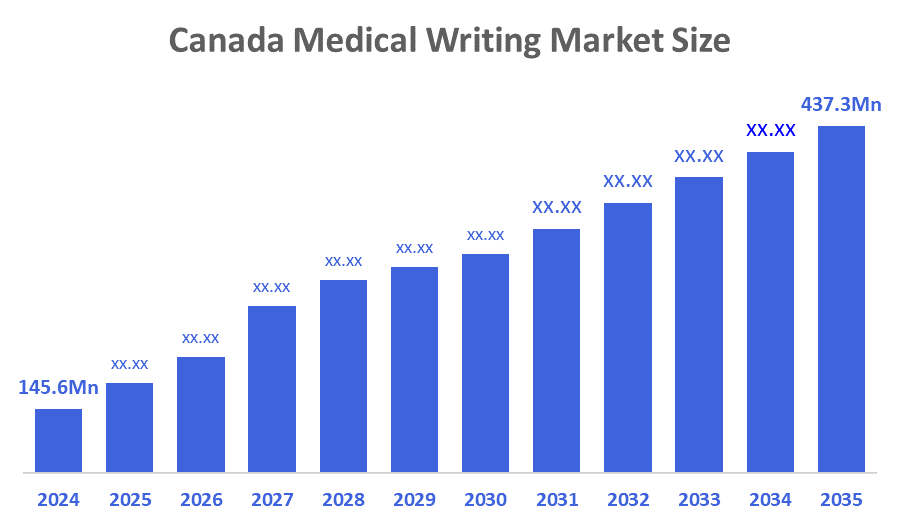
According To a Research Report Published By Decisions Advisors & Consulting, The Canada Medical Writing Market Size Is Anticipated To Hold USD 437.3 Million By 2035, Growing At a CAGR of 10.51% From 2025 to 2035. Future opportunities in the Canada medical writing market include growing demand for regulatory documentation, clinical trial reporting, scientific publications, personalized healthcare content, outsourcing by pharmaceutical companies, and adoption of AI-driven writing and editing tools.
Market Overview
Medical writers in Canada produce documents that support the pharmaceutical, biotechnology and healthcare industry in scientific, regulatory and clinical matters. This includes clinical study reports, manuscripts, regulatory submissions, protocols, patient education materials and medical marketing materials. An increase in the number of clinical trials, as well as a greater number of stringent regulations and a demand for accurate, high quality medical writing will continue to drive the market. Increasing numbers of pharmaceutical companies are outsourcing their medical writing services to comply with regulations, reduce costs and be able to submit documents in a timely manner. Advances in technology will contribute to an increase in productivity through the use of AI-based writing software, electronic systems to create & manage documents, etc. Strong healthcare and pharmaceutical sectors coupled with skilled medical writers and supportive government programs will provide a solid foundation for the continued growth and acceptance of medical writing services in Canada.
Report Coverage
This research report categorizes the market for the Canada medical writing market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Canada medical writing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Canada medical writing market.
Driving Factors
Clinical trials and regulatory demand drive medical writing growth.
The Canada medical writing market is driven by increasing clinical trials, growing pharmaceutical and biotechnology sectors, and stringent regulatory requirements for accurate documentation. Rising outsourcing of medical writing services enhances efficiency, cost-effectiveness, and compliance. Technological advancements, including AI-assisted writing and document management systems, streamline content creation and review. Additionally, the need for high-quality scientific publications, regulatory submissions, and patient education materials fuels market growth. Availability of skilled medical writers and favorable government policies further support expansion in Canada.
Restraining Factors
Skilled workforce shortage and regulatory challenges limit market growth.
High dependence on skilled professionals, stringent regulatory compliance, data confidentiality concerns, and the complexity of medical content creation restrain growth in the Canada medical writing market, limiting adoption among smaller organizations.
Opportunities
Outsourcing and AI tools offer significant medical writing opportunities.
Opportunities in the Canada medical writing market include increasing outsourcing by pharmaceutical and biotechnology companies, rising demand for regulatory and clinical documentation, and growth in scientific publications. Adoption of AI-powered writing tools, e-document management systems, and digital collaboration platforms can enhance efficiency and accuracy. Expansion into specialized therapeutic areas, personalized medicine documentation, and patient-focused educational content presents additional growth potential. Furthermore, the growing clinical research landscape in Canada and emphasis on timely, compliant submissions provide significant prospects for professional medical writing services.
Market Segmentation
The Canada medical writing market share is classified into type and application.
- The regulatory writing segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Canada medical writing market is segmented by type into clinical writing, regulatory writing, scientific writing, and others. Among these, the regulatory writing segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Regulatory writing dominates due to stringent compliance requirements, increasing clinical trials, rising demand for accurate submissions to health authorities, and pharmaceutical companies outsourcing documentation to ensure timely, high-quality regulatory approvals and adherence to evolving Canadian and international guidelines.
- The medical education segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Canada medical writing market is segmented by application into medical journalism, medical education, medico marketing, and others. Among these, the medical education segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Medical education leads due to increasing demand for accurate training materials, e-learning content, clinical guidelines, and educational resources for healthcare professionals, driving the need for high-quality medical writing services in Canada’s expanding healthcare and pharmaceutical sectors.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Canada medical writing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Cactus Inc Class A
- Indegene
- Certara Inc Ordinary Shares
- PAREXEL
- IQVIA Holdings Inc
- Labcorp Drug Development
- FREYR Battery Inc
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Europe, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the Canada medical writing market based on the following segments:
Canada Medical Writing Market, By Type
- Clinical Writing
- Regulatory Writing
- Scientific Writing
- Others
Canada Medical Writing Market, By Application
- Medical Journalism
- Medical Education
- Medico Marketing
- Others
FAQ’s
Q: What is the Canada medical writing market size?
A: Canada medical writing market size is expected to grow from USD 145.6 Million in 2024 to USD 437.3 Million by 2035, growing at a CAGR of 10.51% during the forecast period 2025-2035.
Q: What are the key growth drivers of the market?
A: The Canada medical writing market is driven by increasing clinical trials, growing pharmaceutical and biotechnology sectors, and stringent regulatory requirements for accurate documentation.
Q: What factors restrain the Canada medical writing market?
A: High dependence on skilled professionals, stringent regulatory compliance, data confidentiality concerns, and the complexity of medical content creation restrain growth in the Canada medical writing market, limiting adoption among smaller organizations.
Q: How is the market segmented by type?
A: The market is segmented into type into clinical writing, regulatory writing, scientific writing, and others.
Q: Who are the key players in the Canada medical writing market?
A: Key companies include Cactus Inc Class A, Indegene, Certara Inc Ordinary Shares, PAREXEL, IQVIA Holdings Inc, Labcorp Drug Development, and FREYR Battery Inc.
Q: Who are the target audiences for this market report?
A: The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 250 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Dec 2025 |
| Access | Download from this page |
