Canada Pharmaceutical Microbiology QC Testing Market
Canada Pharmaceutical Microbiology QC Testing Market Size, Share, and COVID-19 Impact Analysis, By Product (Instruments, Reagents & Kits), By Technique (Traditional/Conventional Testing, Growth-based Testing, Nucleic Acid-based Testing, Cellular Component-based Testing, Viability-based Testing, and Other Techniques), and Canada Pharmaceutical Microbiology QC Testing Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Canada Pharmaceutical Microbiology QC Testing Market Size Insights Forecasts to 2035
- The Canada Pharmaceutical Microbiology QC Testing Market Size was estimated at USD 68.25 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 10.48% from 2025 to 2035
- The Canada Pharmaceutical Microbiology QC Testing Market Size is Expected to Reach USD 204.36 Million by 2035
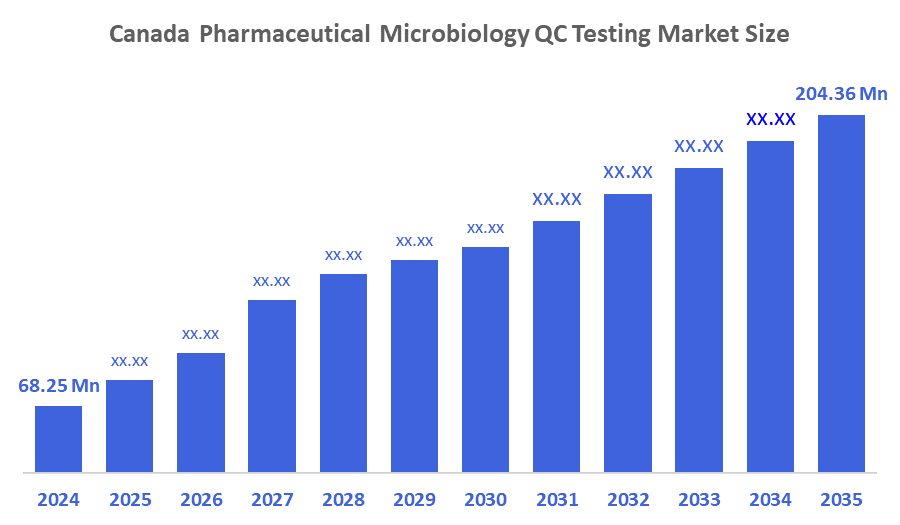
According to a Research Report Published by Decisions Advisors and Consulting, The Canada Pharmaceutical Microbiology QC Testing Market Size is anticipated to Reach USD 204.36 Million by 2035, Growing at a CAGR of 10.48% from 2025 to 2035. Technological developments and a growing focus on patient safety and product quality are the contributing factors to the market's notable expansion.
Market Overview
The Canadian pharmaceutical microbiology QC testing market refers to the section of the pharmaceutical quality control industry that concentrates on microbiological testing techniques to guarantee the safety, sterility, and compliance of drug products. This method of identifying microorganisms at each stage of pharmaceutical production to ensure the product's safety, efficacy, and purity is additionally referred to as pharmaceutical QC. Moreover, strict laws regulating the manufacture of pharmaceuticals and expanding R&D efforts are stimulating the market development. The expanding selection of generic medications and the emphasis on patient safety are additional elements driving market expansion. Additionally, it is anticipated that technological advancements will generate growth opportunities by improving production efficiency and cost-effectiveness. The increasing complexity of biopharmaceutical pipelines, such as those for biologics, sterile injectables, and cell and gene therapies, which necessitate quicker sterility assurance and ongoing microbial monitoring, supports market expansion.
Nearly $134 million was set aside by the Canada Foundation for Innovation (CFI) in 2025 to enhance postsecondary research infrastructure, including microbiology and diagnostic tools necessary for pharmaceutical quality control. More stringent regulations for microbiological testing, Health Canada is actively funding programs to fight antimicrobial resistance and enhance monitoring and access to cutting-edge treatments.
Report Coverage
This research report categorises the market for the Canada pharmaceutical microbiology QC testing market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Canada pharmaceutical microbiology QC testing market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Canada pharmaceutical microbiology QC testing market.
Driving Factors
The fast growth of the biopharmaceutical industry, which necessitates stringent microbiological quality control and environmental monitoring to guarantee product safety and compliance, is the main factor driving the Canadian pharmaceutical microbiology QC testing market. Pharmaceutical companies are forced to invest in advanced microbiological testing services due to strict regulatory standards and growing consumer awareness of product safety. Furthermore, robust and quick microbiological testing solutions are needed for the expanding global research and development efforts, particularly in biologics and novel drug formulations. Also, improving accuracy, efficiency, and technological innovations like automated testing platforms, PCR-based methods, and rapid microbial detection techniques are further accelerating market growth.
In May 2024, Eurofins BioPharma Product Testing (BPT) expanded its biopharma testing services in Canada, with a new facility in Toronto. Eurofins BioPharma Product Testing (EBPT) Canada, to its North American group of companies, EBPT broadens its geographic reach and enhances its capacity for product testing and release services.
Restraining Factors
The Canadian pharmaceutical microbiology QC testing market is constrained by high prices, a presence of skilled workers, complicated regulations, and reliance on imports, all of which limit its ability to grow and compete. These difficulties raise costs, slow down operations, and impede industry innovation.
Market Segmentation
The Canada pharmaceutical microbiology QC testing market share is classified into product and technique.
- The reagents & kits segment dominated the market in 2024 and is projected to grow at a significant CAGR during the forecast period.
The Canada pharmaceutical microbiology QC testing market is differentiated by product into instruments, reagents & kits. Among these, the reagents & kits segment dominated the market in 2024 and is projected to grow at a significant CAGR during the forecast period. Growing biologics and cell and gene therapy pipelines, which have shorter timelines and greater contamination risks, are the main drivers of its expansion. Lyophilised assay kits, endotoxin-free reagents optimised for high-throughput and in-process testing, and cartridge-based systems are in greater demand.
- The growth-based testing segment held a substantial share in 2024 and is expected to grow at a remarkable CAGR during the forecast period.
The Canada pharmaceutical microbiology QC testing market is divided by technique into traditional/conventional testing, growth-based testing, nucleic acid-based testing, cellular component-based testing, viability-based testing, and other techniques. Among these, the growth-based testing segment held a substantial share in 2024 and is expected to grow at a remarkable CAGR during the forecast period. The segment growth is supported by solid regulatory acceptance, dependability, and validation in environmental, bioburden, and testing for sterility testing. Further, manufacturers remain reliant on these proven systems for their accuracy, wide organism detection, and adherence to pharmacopeial standards.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the Canada pharmaceutical microbiology QC testing market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Innovotech Inc.
- Vivariant Laboratories
- CAL Laboratories
- SGS Canada
- SteriLabs
- EMSL Analytical, Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Canada, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the Canada pharmaceutical microbiology QC testing market based on the below-mentioned segments:
Canada Pharmaceutical Microbiology QC Testing Market, By Product
- Instruments
- Reagents & Kits
Canada Pharmaceutical Microbiology QC Testing Market, By Technique
- Traditional/Conventional Testing
- Growth-based Testing
- Nucleic Acid-based Testing
- Cellular Component-based Testing
- Viability-based Testing
- Other Techniques
FAQ
Q: What is the current and projected market size of the Canadian pharmaceutical microbiology QC testing market?
A: The market size was estimated at USD 68.25 million in 2024 and is expected to reach USD 204.36 million by 2035, growing at a CAGR of 10.48% from 2025 to 2035.
Q: What products dominate the market?
A: The reagents & kits segment dominated the market in 2024 and is projected to grow significantly, driven by the demand for biologics and advanced testing kits optimised for high-throughput and contamination risk management.
Q: What testing techniques are used in this market?
A: Techniques include traditional/conventional testing, growth-based testing, nucleic acid-based testing, cellular component-based testing, viability-based testing, and others. Growth-based testing holds a substantial share, chiefly due to its reliability and regulatory acceptance.
Q: What are the key drivers for market growth?
A: Key drivers include rapid growth of the biopharmaceutical industry, stringent microbiological quality control requirements, an increase in R&D activities, technological innovations like automated testing platforms and PCR-based methods, and a rising focus on patient safety and product quality.
Q: What recent developments have impacted the market?
A: In May 2024, Eurofins BioPharma Product Testing expanded its biopharma testing services in Canada with a new facility in Toronto, enhancing testing capacity and geographic reach.
Q: Who are some key players in the Canadian pharmaceutical microbiology QC testing market?
A: Key companies include Innovotech Inc., Vivariant Laboratories, CAL Laboratories, SGS Canada, SteriLabs, and EMSL Analytical, Inc.
Q: What are the main applications of microbiology QC testing in pharmaceuticals?
A: Applications focus on sterility testing, contamination control in biologics, monitoring bioburden levels, and ensuring safety and efficacy throughout pharmaceutical production processes.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 285 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
