Global Cell and Gene Therapy Manufacturing QC Market
Global Cell and Gene Therapy Manufacturing QC Market Size, Share, and COVID-19 Impact By Therapy Type (Cell Therapy [CAR-T {Autologous CAR-T, Allogeneic CAR-T}, CAR-NK, B-Cell, & Others], and Gene Therapy [Viral {AAV, Lenti, and Other}, Non-Viral]), By Scale (Pre-commercial/ R&D Manufacturing, Commercial Scale Manufacturing), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Report Overview
Table of Contents
Global Cell and Gene Therapy Manufacturing QC Market Overview
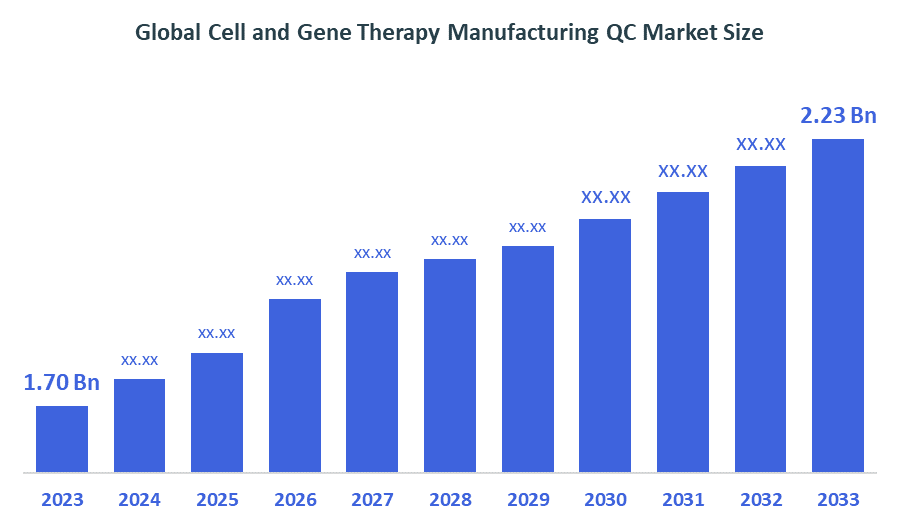
- The Global Cell and Gene Therapy Manufacturing QC Market Size was worth around USD 1.70 Billion in 2023 and is Predicted to Grow to around USD 2.23 Billion By 2033 with a compound annual growth rate (CAGR) Of 2.75% Between 2023 and 2033.
- The market growth is rising due to are growing pipeline of cell & gene therapy (CGT), increasing investments in advanced therapy medicinal products (ATMPs) sector including cell & gene therapy, and an increasing number of CAR-T cell therapy trials.
- The cell and gene therapy manufacturing QC market refers to the industry that the group of testing and verification activities employed to assure the safety, purity, identity, potency, and quality of cell and gene therapy products at and after the time of manufacturing.
Major vendors in the Global Cell and Gene Therapy Manufacturing QC Market
WuXi AppTec, Lonza, Thermo Fisher Scientific, Charles River Laboratories, Catalent, Merck KGaA, Eurofins Scientific, Pace, Pharmaron, BioAgilytix Labs, Avance Biosciences, SGS, and others.
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2023 to 2033. Decision Advisors has segmented the cell and gene therapy manufacturing QC market based on the below-mentioned segments:
Global Cell and Gene Therapy Manufacturing QC Market, By Therapy Type
- (Cell Therapy [CAR-T {Autologous CAR-T, Allogeneic CAR-T}
- CAR-NK
- B-Cell
- Others
Global Cell and Gene Therapy Manufacturing QC Market, By Gene Therapy
- Viral {AAV, Lenti, and Other}
- Non-Viral
Global Cell and Gene Therapy Manufacturing QC Market, By Scale
- Pre-commercial/ R&D Manufacturing
- Commercial Scale Manufacturing
Global Cell and Gene Therapy Manufacturing QC Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Global |
| Pages | 250 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Aug 2025 |
| Access | Download from this page |
