France Clinical Trials Management System Market
France Clinical Trials Management System Market Size, Share, and COVID-19 Impact Analysis, By Delivery Code (Web & Cloud-Based and On-Premise), By Component (Software and Services), By Solution Type (Enterprise and Site), By End User (Pharmaceutical & Biotechnology Firms, Medical Device Firms, CROs, and Others), and France Clinical Trials Management System Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
France Clinical Trials Management System Market Size, Share, and COVID-19 Impact Analysis, By Delivery Code (Web & Cloud-Based and On-Premise), By Component (Software and Services), By Solution Type (Enterprise and Site), By End User (Pharmaceutical & Biotechnology Firms, Medical Device Firms, CROs, and Others), and France Clinical Trials Management System Market Insights, Industry Trend, Forecasts to 2035
France Clinical Trials Management System Market Insights Forecasts to 2035
- The France Clinical Trials Management System Market Size Was Estimated at USD 75.9 Million in 2024.
- The France Clinical Trials Management System Market Size is Expected to Grow at a CAGR of Around 12.9% from 2025 to 2035.
- The France Clinical Trials Management System Market Size is Expected to Reach USD 287.9 Million by 2035.
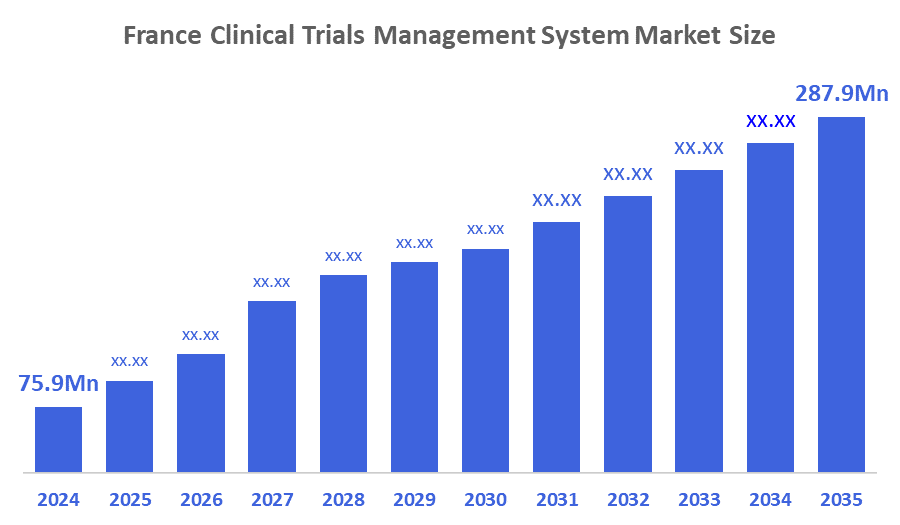
According To a Research Report Published By Decisions Advisors & Consulting, The France Clinical Trials Management System Market Size Is Anticipated To Reach USD 287.9 Million By 2035, Growing At a CAGR Of 12.9% From 2025 to 2035. The France clinical trials management system market is driven by the growing number and complexity of clinical trials, increased adoption of digital and cloud-based solutions, and the need for efficient data management and regulatory compliance. Strong government support for clinical research, rising use of decentralized and patient-centric trials, and demand for real-time monitoring and operational efficiency further contribute to market growth.
Market Overview
The?????? France clinical trials management system market describes the market for software solutions utilized in France to schedule, follow up, handle, and make clinical trial operations more efficient. Such systems are a source of support for sponsors, contract research organizations (CROs), and research institutions, since they manage trial activities like site management, patient enrollment, budgeting, compliance, data tracking, and reporting, at the same time, they ensure that the regulations of France and Europe are ??????respected.
France faces a substantial and growing public health burden from chronic diseases and aging, creating a strong need for efficient clinical research and patient-centric care systems. Over 20 million people in France suffer from chronic conditions such as cardiovascular disease, diabetes, and neurological disorders, and the prevalence of these conditions is rising with an aging population. In 2021, about 12 million patients were officially recognized with a long-term chronic disease, and chronic illness rates have increased over time. Additionally, a significant portion of the adult population reports long-term health problems; particularly among older age groups, over half of people aged 50 and above have chronic or long-lasting health issues.
France is also very active in clinical research, initiating thousands of clinical trials and maintaining its position as a leading European country in trial activity and authorization submissions. This combination of high disease burden, complex health needs, and extensive research activity underscores the importance of robust clinical trial management system solutions to streamline trial planning, patient recruitment, data tracking, regulatory compliance, and overall trial efficiency in the France healthcare and research ecosystem.
Report Coverage
This research report categorizes the France clinical trials management system market based on various segments and regions, forecasting revenue growth and analyzing trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France clinical trials management system market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France clinical trials management system market.
Driving Factors
The?????? France clinical trials management system market is driven by the increasing volume of clinical trials, which are also becoming more complex, and the rising use of digital and cloud-based solutions to enhance efficiency. Tough regulatory and compliance requirements in France and the EU are the main reasons for the adoption of organized trial management platforms. Besides that, the government backing research, the increase in decentralized and patient-centric trials, as well as the necessity for real-time data access, cost control, and improved coordination between sponsors, CROs, and research sites, are some of the factors that are still leading to the market ??????expansion.
Restraining Factors
The?????? France clinical trials management system market is restrained by the expensive implementation and maintenance of a CTMS software condition, especially for research organizations with small and medium-sized groups. Security and privacy issues exacerbate the situation because the adoption of such systems is limited due to strict regulations like GDPR. The difficulties encountered in the integration of clinical and hospital IT systems already exist, the resistance of the staff to the standard method of trial management, and the requirement of qualified persons to operate and manage the CTMS platform are a few factors that limit the expansion of the market. ??????
Market Segmentation
The France clinical trials management system market share is categorized by delivery code, component, solution type, and end user.
- The web & cloud-based segment accounted for the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France clinical trials management system market is segmented by delivery code into web & cloud-based and on-premise. Among these, the web & cloud-based segment accounted for the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The web & cloud-based segment's growth is due to its scalability, lower implementation costs, real-time data access, easier collaboration, and strong support for decentralized trials and regulatory compliance, making it more flexible and efficient than on-premise systems.
- The software segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
The France clinical trials management system market is segmented by component into software and services. Among these, the software segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The software’s segmental growth is driven by the increasing adoption of advanced CTMS platforms to manage complex clinical trial workflows, rising demand for real-time data tracking and analytics, the need for regulatory compliance, and the growing use of digital and cloud-based trial management solutions across sponsors, CROs, and research institutions.
- The enterprise segment accounted for the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France clinical trials management system market is segmented by solution type into enterprise and site. Among these, the enterprise segment accounted for the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The enterprise segment's growth is due to its ability to manage large-scale, multi-site clinical trials efficiently, provide centralized data management and real-time analytics, ensure regulatory compliance across sites, and support better collaboration among sponsors, CROs, and research institutions. Its scalability and integration capabilities make it ideal for organizations conducting complex and extensive clinical research.
- The pharmaceutical & biotechnology firms segment held the largest market share in 2024 and is projected to grow at a substantial CAGR during the forecast period.
The France clinical trials management system market is segmented by end user into pharmaceutical & biotechnology firms, medical device firms, CROs, and others. Among these, the pharmaceutical & biotechnology firms segment held the largest market share in 2024 and is projected to grow at a substantial CAGR during the forecast period. The pharmaceutical & biotechnology firms’ segmental growth is due to the increasing number of drug development programs, rising R&D investments, and the need for efficient management of complex clinical trials. These firms rely heavily on CTMS solutions for patient recruitment, regulatory compliance, real-time data tracking, and streamlined trial operations across multiple sites, driving strong adoption of these systems.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France clinical trials management system market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Medidata
- IQVIA Inc.
- Oracle Corporation
- Clario
- DATATRAK International, Inc.
- SimpleTrials
- RealTime Software Solutions, LLC
- Wipro Limited
- PHARMASEAL International Limited
- Veeva Systems Inc.
- Peachtree BioResearch Solutions
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In February 2021, Veeva Systems (NYSE: VEEV) announced that 6 out of the top 7 global CROs have joined the Veeva Vault CDMS partner program. Accelerating clinical research is a top priority in the life sciences industry. All of the top 7 CROs are now able to leverage Veeva Vault CDMS capabilities to modernize clinical data management and build high-quality studies faster.
Market Segment
This study forecasts revenue at the France, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the France Clinical Trials Management System Market based on the below-mentioned segments:
France Clinical Trials Management System Market, By Delivery Code
- Web & Cloud-Based
- On-Premise
France Clinical Trials Management System Market, By Component
- Software
- Services
France Clinical Trials Management System Market, By Solution Type
- Enterprise
- Site
France Clinical Trials Management System Market, By End User
- Pharmaceutical & Biotechnology Firms
- Medical Device Firms
- CROs
- Others
Frequently Asked Questions (FAQ)
- What is the France clinical trials management system market size in 2024?
The France clinical trials management system market size was estimated at USD 75.9 million in 2024.
- What is the projected market size of the France clinical trials management system market by 2035?
The France clinical trials management system market size is expected to reach USD 287.9 million by 2035.
- What is the CAGR of the France clinical trials management system market?
The France clinical trials management system market size is expected to grow at a CAGR of around 12.9% from 2024 to 2035.
- What are the key growth drivers of the France clinical trials management system market?
Increased adoption of digital and cloud-based solutions, and the need for efficient data management and regulatory compliance. Strong government support for clinical research, rising use of decentralized and patient-centric trials.
- Which component segment dominated the market in 2024?
The software segment dominated the market in 2024.
- Which delivery code segment accounted for the largest market share in 2024?
The web & cloud-based segment accounted for the largest market share in 2024.
- What segments are covered in the France clinical trials management system market report?
The France clinical trials management system market is segmented on the basis of delivery code, component , solution type, and end user.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Regional |
| Pages | 250 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Dec 2025 |
| Access | Download from this page |
