France Neurology Devices Market
France Neurology Devices Market Share, Size, and COVID-19 Impact Analysis, By Device Type (Cerebrospinal Fluid Management Devices, Interventional Neurology Devices, Neurosurgery Devices, Neurostimulation Devices, and Others), By End-User (Hospitals and Clinics, Research and Academic Institutions, Home Care Settings, Ambulatory Surgical Centers, and Others), and France Neurological Devices Market Insights, Industry Trends, Forecasts to 2035.
Report Overview
Table of Contents
France Neurology Devices Market Insights Forecasts to 2035
- The France Neurology Devices Market Size Was Estimated at USD 2.97 Billion in 2024.
- The France Neurology Devices Market Size is Expected to Grow at a CAGR of Around 8.2% from 2025 to 2035.
- The France Neurology Devices Market Size is Expected to Reach USD 7.07 Billion by 2035.
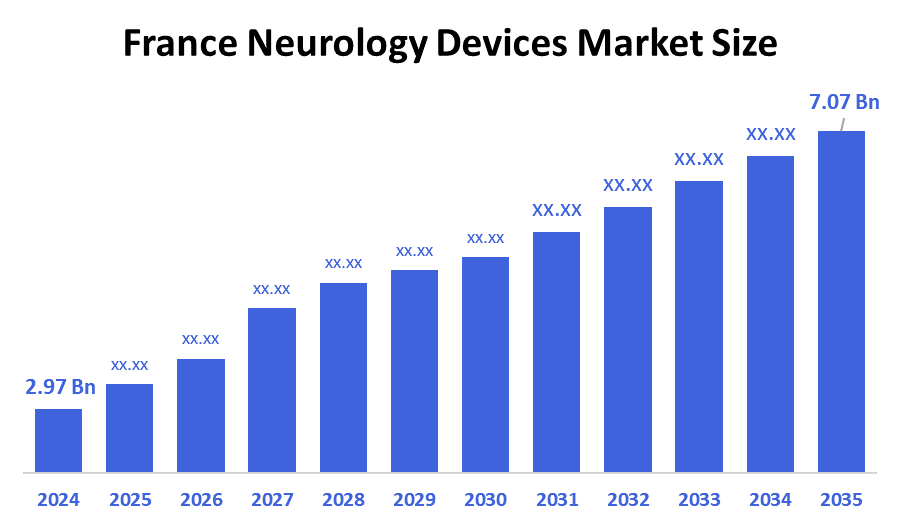
According to a research report published by decision advisor & Consulting, the France Neurological Devices Market Size is projected to reach USD 7.07 billion by 2035, growing at a CAGR of 8.2% from 2025 to 2035. The France neurological devices market is driven by the increasing prevalence of neurological diseases, particularly among the aging population, and technological advancements that lead to more effective diagnostic and therapeutic devices. Other key drivers include strategic initiatives by market players to develop innovative products.
Market Overview
The France neurology devices market refers to the commercial market for all medical tools and equipment used in France to diagnose, monitor, treat, and research neurological disorders that affect the central and peripheral nervous systems. This includes a wide range of products such as neurostimulation devices, interventional neurology devices, CSF management systems, and neurosurgery equipment, which are utilized by healthcare professionals and hospitals within the country. The France neurology devices market is driven by a high prevalence of neurological disorders, particularly among an aging population susceptible to degenerative diseases like Alzheimer's and Parkinson's.
The increased healthcare expenditure and R&D investment, a growing demand for diagnostic and therapeutic solutions, and technological advancements are leading to innovative and minimally invasive devices through the integration of cutting-edge technologies like artificial intelligence (AI), robotics, advanced imaging, and 3D printing. These innovations aim to improve diagnostic accuracy, enhance surgical precision, and reduce patient recovery times.
Neurosurgery devices in France are expected to experience significant growth due to the rising prevalence of neurological disorders. For example, a PubMed study published in January 2022 highlighted traumatic brain injury (TBI) as a major public health concern in France, affecting young patients, road accident victims, the elderly, and fall victims. The need for intracranial pressure monitors to manage TBI is expected to drive demand for neurological devices. Additionally, the Alzheimer’s Association notes that individuals with severe TBI have a 4.5-times higher risk of developing cognitive disorders such as dementia, further supporting market growth.
Report Coverage
This research report categorizes the market for the France neurology devices market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France neurology devices market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France neurology devices market.
Driving Factors
The France neurological devices market is driven by an aging population in France is leading to a higher incidence of neurological disorders such as Alzheimer's disease, Parkinson's disease, and stroke, creating significant demand for advanced diagnostic and therapeutic devices. Furthermore, increased healthcare spending, with advancements in neurology device technology, such as minimally invasive surgical tools and sophisticated neuro-stimulation devices, is driving market growth. Technological innovation, including the development of AI-powered diagnostic tools and personalized treatment approaches, further contributes to market growth.
Restraining Factors
The high cost of advanced neurological devices is one of the restraining factors for the France neurology device market. Many devices, especially Deep Brain Stimulation (DBS) and Magnetic Resonance Imaging (MRI) systems, are expensive, which can limit accessibility for some patients, the time-consuming regulatory approval process, and the limited number of skilled neurosurgeons and healthcare professionals. Adoption of complex equipment can be discouraged in smaller healthcare facilities due to the high initial investment and ongoing maintenance costs.
Market Segmentation
The France neurological devices market share is categorized by device type and end user.
- The neurostimulation devices segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France neurological devices market is segmented by device type into cerebrospinal fluid management devices, interventional neurology devices, neurosurgery devices, neurostimulation devices, and others. Among these, the neurostimulation devices segment accounted for the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The neurostimulation devices segmental growth is due to their effectiveness in treating chronic pain and movement disorders, the increasing prevalence of these conditions, technological advancements, and growing patient and physician preference for these durable and less invasive treatment options. These factors drive the market, as companies focus on launching new products for therapies like deep brain stimulation (DBS) and spinal cord stimulation.
- The hospitals and clinics held the largest market share in 2024 and are anticipated to grow at a significant CAGR during the forecast period.
The France neurological devices market is segmented by end user into hospitals and clinics, research and academic institutions, home care settings, ambulatory surgical centers, and others. Among these, the hospitals and clinics held the largest market share in 2024 and are anticipated to grow at a significant CAGR during the forecast period. This segmental growth is due to their role in handling acute and chronic neurological conditions, the presence of advanced diagnostic tools, and their capacity for specialized, high-volume treatments. These facilities are equipped to perform complex procedures and manage patients with time-sensitive emergencies, which drives demand for a wide range of neurological devices.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France neurological devices market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Medtech (FRA)
- Xenios
- In Vivo Neurotech
- NeuroMed Technologie
- Medtronic
- Boston Scientific
- Abbott Laboratories
- Johnson & Johnson
- Stryker
- Philips Healthcare
- Siemens Healthineers
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
In February 2025, Medtronic received U.S. FDA approval for its BrainSense Adaptive Deep Brain Stimulation (ADBS) system, the world’s first adaptive DBS therapy for Parkinson’s disease. The system continuously monitors brain activity and automatically adjusts stimulation in real time. Built on the Percept neurostimulator platform, it delivers highly personalized, precise treatment. This marks a major milestone in Parkinson’s care and the largest commercial launch of brain–computer interface technology to date.
In November 2024, Royal Philips and iCometrix announced a partnership to deploy an AI-driven MRI brain imaging solution unveiled at RSNA 2024. The system integrates Icometrix’s AI software with Philips BlueSeal MR scanners for advanced neurological diagnosis. It enhances the detection and monitoring of conditions like Alzheimer’s disease and multiple sclerosis (MS).
Market Segment
This study forecasts revenue at the France, regional, and country levels from 2020 to 2035.decision advisor has segmented the France Neurological Devices Market based on the below-mentioned segments: France Neurological Devices Market, By Device Type
- Cerebrospinal Fluid Management Devices
- Interventional Neurology Devices
- Neurosurgery Devices
- Neurostimulation Devices
- Others
France Neurological Devices Market, By End User
- Hospitals and Clinics
- Research and Academic Institutions
- Home Care Settings
- Ambulatory Surgical Centers
- Others
Frequently Asked Questions (FAQ)
- What is the CAGR of the France neurological devices market?
The France neurological devices market size is expected to grow at a CAGR of around 8.2% from 2024 to 2035
- What is the France neurological devices market size in 2024?
The France neurological devices market size was estimated at USD 2.97 billion in 2024.
- What is the projected market size of the France neurological devices market by 2035?
The France neurological devices market size is expected to reach USD 7.07 billion by 2035
- What are the main segments of the France neurological devices market?
The France neurological devices market share is segmented based on device type and end user.
- What are some drivers contributing to market growth?
France neurological devices market is driven by aging population, rising cases of disorders like Alzheimer’s and Parkinson’s, increased healthcare spending, and advances in technologies such as minimally invasive tools, neurostimulation devices, and AI-powered diagnostics.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | country |
| Pages | 176 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
