France Rare Diseases Treatment Market
France Rare Diseases Treatment Market Size, Share, and COVID-19 Impact Analysis, By Therapeutic Area (Cancer, Neurological Conditions, Cardiovascular Conditions, Metabolic Diseases, and Others), By Drug Type (Biologics, Biosimilars, Small molecules, and Others), By Route of Administration (Oral and Injectable), By Distribution Channel (Hospital Pharmacy, Specialty Pharmacy, Online Pharmacy, and Others), and France Rare Diseases Treatment Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
France Rare Diseases Treatment Market Insights Forecasts to 2035
- The France Rare Diseases Treatment Market Size Was Estimated at USD 4,449.9 Million in 2024
- The France Rare Diseases Treatment Market Size is Expected to Grow at a CAGR of Around 12.98% from 2025 to 2035
- The France Rare Diseases Treatment Market Size is Expected to Reach USD 17,028.6 Million by 2035
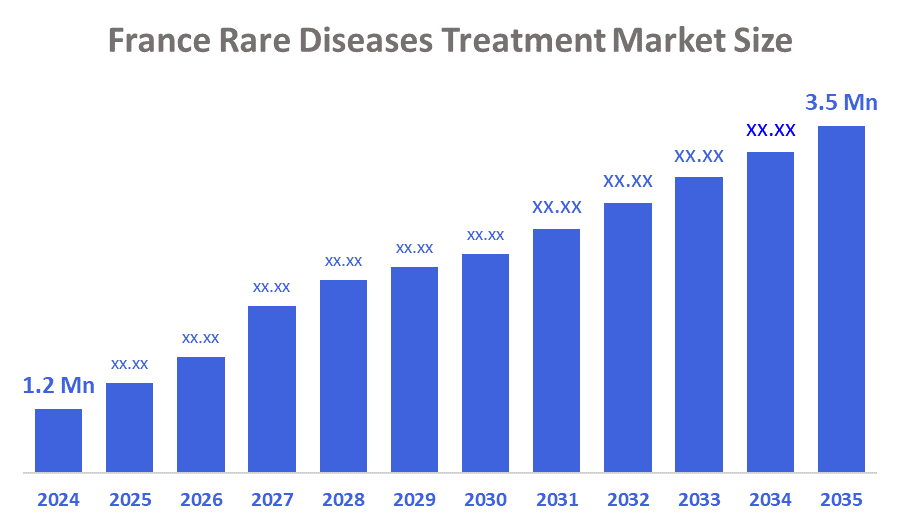
According to a research report published by Decision Advisor & Consulting, The France Rare Diseases Treatment Market Size is anticipated to reach USD 17,028.6 Million by 2035, Growing at a CAGR of 12.98% from 2025 to 2035. The France rare diseases treatment market is driven by the growing number of diagnosed rare disease patients, high unmet medical needs, and better awareness and early diagnosis. Strong government support, reimbursement policies, and advances in biotechnology and orphan drug development also support market growth.
Market Overview
The rare diseases treatment market in France encompasses activities related to the development, production, distribution, and use of specialized therapies for rare diseases affecting fewer than 1 in 2,000 people. The market mainly consists of orphan drugs, biologics, enzyme replacement, and advanced gene and cell therapies. These innovative treatments are supported by strong government policies, reimbursement systems, and specialized healthcare centers to ensure patient access.
France's rare diseases treatment market is largely determined by population health needs and government engagement. Rare diseases collectively affect over 3 million people in France, which is around 4.5% of the population, through over 7,000 identified conditions. A large number of these diseases are serious, long-lasting, and usually of genetic origin, and about 95% of them still do not have effective treatment options, thus pointing out the huge unmet medical need and the importance of rare disease therapies. In many instances, patients undergo diagnostic delays of five years or more, and therefore, giving them timely access to specialized treatments and care becomes a vital public health issue.
France has built a well-organized rare diseases ecosystem in Europe with the help of different National Rare Diseases Plans (PNMR) to tackle these problems. The recent 4th Plan National Maladies Rares (2025-2030) allocates nearly €223 million yearly to enhance patient care, decrease diagnostic delays, and facilitate research collaborations among clinicians, researchers, and patient groups. The plan also envisages extending access to specialized care through more than 600 rare disease reference centers spread across the country, supported by money allocated (at least €36 million for the new center accreditation) for strengthening the expert networks. These initiatives reflect a sustained commitment to innovation and treatment development.
Report Coverage
This research report categorizes the market for the France rare diseases treatment market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France rare diseases treatment market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France rare diseases treatment market.
Driving Factors
The France rare diseases treatment market is driven by several factors, such as a large number of people suffering from rare diseases, a handful of treatment options, and a robust government support system. The diagnosis of diseases at an early stage, the use of novel technology like gene therapy, and a vibrant research and patient awareness environment have resulted in an increased demand for efficacious rare disease treatments.
Restraining Factors
The France rare diseases treatment market is restrained by the high cost of treatments, the small number of patients, and the long time it takes to develop drugs. Moreover, strict pricing and reimbursement regulations, delays in market access approvals, and complicated clinical trials slow down the adoption of new treatments, even though there is a strong medical need for them.
Market Segmentation
The France rare diseases treatment market share is classified into therapeutic area, drug type, route of administration, and distribution channel.
- The cancer segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France rare diseases treatment market is segmented by therapeutic area into cancer, neurological conditions, cardiovascular conditions, metabolic diseases, and others. Among these, the cancer segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The cancer segment is growing because rare cancers are becoming more commonly diagnosed, there is a strong research focus, and new targeted and gene-based treatments are being developed with government support, leading to higher use of cancer therapies.
- The biologics segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period.
The France rare diseases treatment market is segmented by drug type into biologics, biosimilars, small molecules, and others. Among these, the biologics segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period. The biologics segment is growing because biologic drugs are highly effective for treating complex, rare diseases, especially genetic and immune-related conditions. Increased use of targeted therapies and gene and enzyme replacement treatments, along with strong research investment and government support, is driving higher adoption of biologics in France.
- The injectable segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period.
The France rare diseases treatment market is segmented by route of administration into oral and injectable. Among these, the injectable segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period. The injectable segment is growing because many rare disease treatments, especially biologics and gene therapies, can only be given by injection, which ensures they work effectively, and there is increasing use of advanced injectable therapies in France.
- The specialty pharmacy segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France rare diseases treatment market is segmented by distribution channel into hospital pharmacy, specialty pharmacy, online pharmacy, and others. Among these, the specialty pharmacy segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. The specialty pharmacy segment is growing because these pharmacies provide access to complex and high-cost rare disease treatments, offer expert guidance and patient support, and help ensure safe and effective use of advanced therapies in France.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France rare diseases treatment market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Sanofi SA
- Roche Holding AG
- Novartis AG
- Pfizer Inc.
- AstraZeneca (Alexion)
- AbbVie Inc.
- Bayer AG
- Bristol-Myers Squibb
- Amgen Inc.
- Orphan Europe
- Ipsen
- Orphalan S.A.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments:
- In January 2025, France launched its 4th National Rare Diseases Plan (PNMR4), enhancing care, research, and access to treatments for over 3 million patients. The plan expands specialized centers, speeds diagnosis, promotes innovative therapies, and strengthens European collaboration.
- In December 2024, in France, the OrphanDev and PedStart research networks teamed up to build a national framework that speeds up patient access to innovative gene and cell therapies for rare diseases, aiming to boost clinical trials and treatment availability.
Market Segment
This study forecasts revenue at the France, regional, and country levels from 2020 to 2035. Decision Advisor has segmented the France rare diseases treatment market based on the below-mentioned segments:
France Rare Diseases Treatment Market, By Therapeutic Area
- Cancer
- Neurological Conditions
- Cardiovascular Conditions
- Metabolic Diseases
- Others
France Rare Diseases Treatment Market, By Drug Type
- Biologics
- Biosimilars
- Small molecules
- Others
France Rare Diseases Treatment Market, By Route of Administration
- Oral
- Injectable
France Rare Diseases Treatment Market, By Distribution Channel
- Hospital Pharmacy
- Specialty Pharmacy
- Online Pharmacy
- Others
FAQ’s
- What is the France rare diseases treatment market size in 2024?
The France rare diseases treatment market size was estimated at USD 4,449.9 million in 2024.
- What is the projected market size of the France rare diseases treatment market by 2035?
The France rare diseases treatment market size is expected to reach USD 17,028.6 million by 2035.
- What is the CAGR of the France rare diseases treatment market?
The France rare diseases treatment market size is expected to grow at a CAGR of around 12.98% from 2024 to 2035.
- What are the key growth drivers of the France rare diseases treatment market?
The France rare diseases treatment market is driven by the growing number of diagnosed rare disease patients, high unmet medical needs, and better awareness and early diagnosis. Strong government support, reimbursement policies, and advances in biotechnology and orphan drug development also support market growth.
- Which drug-type segment dominated the market in 2024?
The biologics segment dominated the market in 2024.
- What segments are covered in the France rare diseases treatment market report?
The France rare diseases treatment market is segmented on the basis of therapeutic area, drug type, route of administration, and distribution channel.
- Who are the key players in the France rare diseases treatment market?
Key companies include Sanofi SA, Roche Holding AG, Novartis AG, Pfizer Inc., AstraZeneca (Alexion), AbbVie Inc., Bayer AG, Bristol-Myers Squibb, Amgen Inc., Orphan Europe, Ipsen, Orphalan S.A., and others.
- Who are the target audiences for this market report?
The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 240 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Jan 2026 |
| Access | Download from this page |
