Germany Bioprocess Validation Market
Germany Bioprocess Validation Market Size, Share, and COVID-19 Impact Analysis, By Testing Type (Extractables & Leachables Testing and Bioprocess Residuals Testing), By Mode (In house and Outsourced), and Germany Bioprocess Validation Market Insights, Industry Trend, Forecasts to 2035.
Report Overview
Table of Contents
Germany Bioprocess Validation Market Insights Forecasts to 2035
- The Germany Bioprocess Validation Market Size Was Estimated at USD 36.65 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 10.31% from 2025 to 2035
- The Germany Bioprocess Validation Market Size is Expected to Reach USD 107.87 Million by 2035
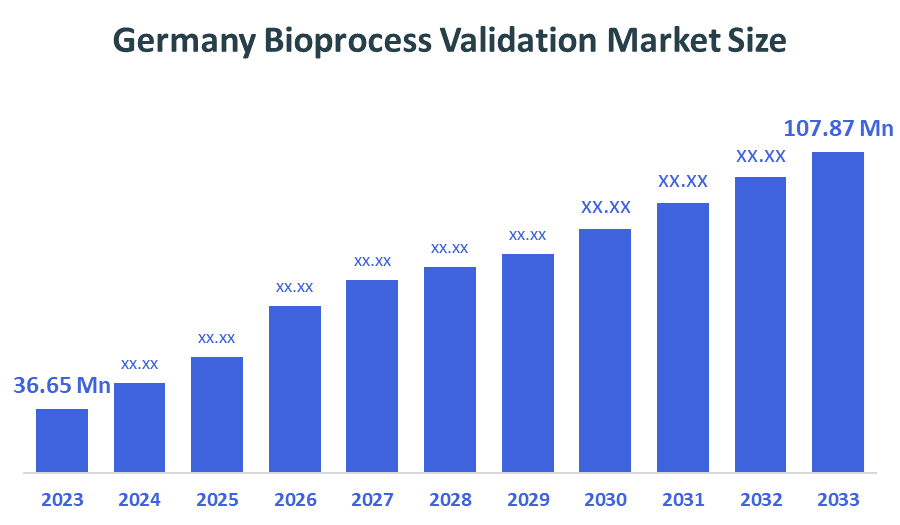
According to a research report published by Spherical Insights & Consulting, The Germany Bioprocess Validation Market is anticipated to reach USD 107.87 Million by 2035, growing at a CAGR of 10.31%from 2025 to 2035. Bioprocess validation is the process of documenting all the procedures, activities, and evidence of the process of biological and biopharmaceutical product formation. The documentation is done as per the US FDA guidelines and cGMP regulations. It ensures the maintenance of compliance in all the stages of the product testing procedure.
Market Overview
The bioprocess validation market focuses on the verification and documentation of biopharmaceutical manufacturing processes to ensure they produce products that meet pre-determined quality standards consistently. This is crucial to validating safety, efficacy and regulatory compliance, and it occupies a significant space in the scope of continued drug development and production. The bioprocess validation market has benefits such as reducing production risk; ensuring improved product consistency; and expediting regulatory approvals. There is expected to be considerable growth opportunities in the market for process optimization, automation, and advanced analytical tools due to the growth in demand for biologics and biosimilars. Critical processes have global government funding and support, regulatory framework upgrades, and incentives geared towards domestic biomanufacturing capabilities. In Germany, government initiatives that foster biotech innovation, compliance to good manufacturing practices (GMP), and identification of partner sources in the public research sector have fueled additional market growth; therefore, enhancing global competitiveness as well as ensuring safe and efficient biologic drugs manufacturing.
Report Coverage
This research report categorizes the market for Germany Bioprocess Validation market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Germany Bioprocess Validation market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment Germany Bioprocess Validation market.
Driving Factors
The increasing demand for biologics, biosimilars, and vaccines, which require the stringent application of high quality and regulatory standards. Rising regulatory scrutiny from regulators, such as the EMA and FDA, drives manufacturers to continue to consistently provide a safe and efficacious product. Advances in bioprocessing technologies and automation have all contributed to the growth of this market.
Restraining Factors
The difficulty and costs associated with validation processes, especially for small and medium-sized enterprises. Regulatory standards are demanding and require time, documentation, and resources. Validating new products mean increased time to market. In addition to the considerable cost and difficultly, there is a shortage of validated services and experienced professionals in the validation market.
Market Segmentation
The Germany bioprocess validation market share is classified into testing type and mode.
- The extractables & leachables testing segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Germanyn bioprocess validation market is segmented by testing type into extractables & leachables testing and bioprocess residuals testing. Among these, the extractables & leachables testing segment held a significant share in 2024 and is expected to grow at a substantial CAGR during the forecast period. The rising regulatory scrutiny and the growing use of single-use systems in biopharmaceutical manufacturing. Ensuring product safety by identifying potential contaminants from packaging and processing materials is critical, driving demand for E&L testing.
- The in-house segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Germany bioprocess validation market is segmented by mode into in house and outsourced. Among these, the in-house segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. The growing investments by biopharmaceutical companies in building internal capabilities for better process control, data security, and regulatory compliance. In-house validation allows faster turnaround times, tailored protocols, and reduced dependency on third parties.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Germany bioprocess validation market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the companies' current news and developments, including product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Hoppecke Batterien
- Lion Smart (LION Smart GmbH)
- Manz AG
- Akasol
- Customcells
- TerraE
- BMZ Group
- VARTA AG
- Tesvolt
- E3/DC
- Skeleton Technologies
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Germany, regional, and country levels from 2020 to 2035. Decision Advisor has segmented the Germany bioprocess validation market based on the below-mentioned segments:
Germany Bioprocess Validation Market, By Testing Type
- Extractables & Leachables Testing
- Bioprocess Residuals Testing
Germany Bioprocess Validation Market, By Mode
- In house
- Outsourced
Frequently Asked Questions
Q.1: What is the market size of the Germany Bioprocess Validation Market in 2024?
A: The Germany Bioprocess Validation Market size was estimated USD 36.65 Million in 2024.
Q.2: What is the forecasted CAGR of the Germany Bioprocess Validation Market from 2024 to 2035?
A: The market is expected to grow at a CAGR of around 10.31% during the period 2024–2030.
Q.3: Who are the top 10 companies operating in the Germany Bioprocess Validation Market?
A: Key players include Hoppecke Batterien, Lion Smart (LION Smart GmbH), Manz AG, Akasol, Customcells, TerraE, BMZ Group, VARTA AG, Tesvolt, E3/DC, Skeleton Technologies, Others
Q.4: What are the main drivers of growth in the Germany Bioprocess Validation Market?
A: The increasing demand for biologics, biosimilars, and vaccines, which require the stringent application of high quality and regulatory standards.
Q.5: What are the main restraining of growth in the Germany Bioprocess Validation Market?
A: The difficulty and costs associated with validation processes, especially for small and medium-sized enterprises.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Global |
| Pages | 235 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Aug 2025 |
| Access | Download from this page |
