Global Hepatic Fibrosis Biomarkers Market
Global Hepatic Fibrosis Biomarkers Market Size, Share, and COVID-19 Impact Analysis, By Product (Kits and Reagents, Assays, Services), By Technology (Immunoassay, Molecular Diagnostics, Proteomics/Metabolomics, Imaging-based Biomarker Assessment), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Report Overview
Table of Contents
Hepatic Fibrosis Biomarkers Market Summary
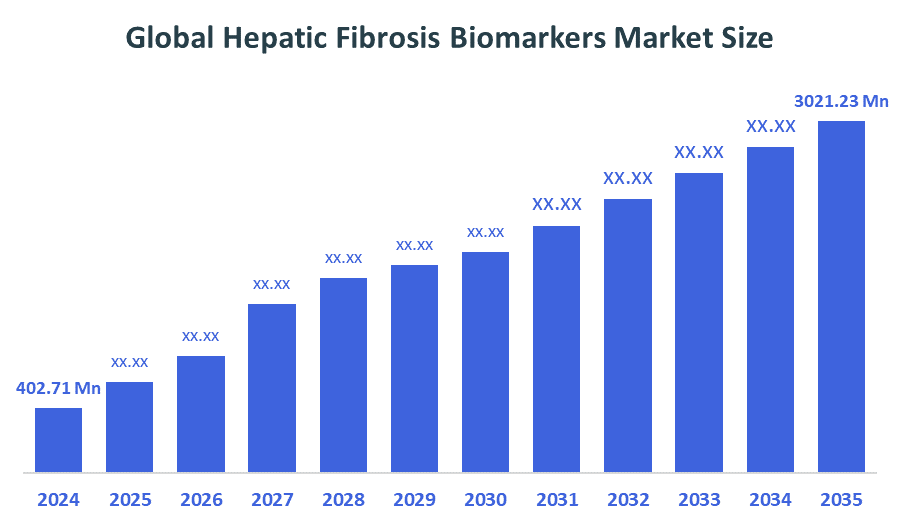
The Global Hepatic Fibrosis Biomarkers Market Size Was Estimated at USD 402.71 Million in 2024 and is Projected to Reach USD 3021.23 Million by 2035, Growing at a CAGR of 20.11% from 2025 to 2035. The growing frequency of liver illnesses like NASH, the limits of liver biopsies, and the growing need for non-invasive diagnostic tools for early identification and monitoring are all contributing factors to the growth of the hepatic fibrosis biomarkers market.
Key Regional and Segment-Wise Insights
- In 2024, North America held the greatest revenue share of 47.36% in the global market for hepatic fibrosis biomarkers.
- With the most revenue share of 47.52% in 2024, the kits and reagents segment dominated the market based on product.
- In terms of technology, the immunoassay segment dominated the market in 2024, holding the biggest revenue share at 43.52%.
Global Market Forecast and Revenue Outlook
- 2024 Market Size: USD 402.71 Million
- 2035 Projected Market Size: USD 3021.23 Million
- CAGR (2025-2035): 20.11%
- North America: Largest market in 2024
The market for hepatic fibrosis biomarkers operates through the development and implementation of biological markers that detect and monitor liver fibrosis as a result of chronic liver disease from hepatitis, nonalcoholic fatty liver disease, NASH, and alcohol-related liver disease. Biomarkers detected through imaging or blood testing enable faster non-invasive diagnostic procedures that replace traditional liver biopsies. The rising global incidence of persistent liver diseases, combined with the requirement for better disease surveillance tools and the increasing desire for prompt diagnosis, drives the market forward. Trustworthy hepatic fibrosis biomarkers become increasingly essential due to the aging population, alongside rising lifestyle risk factors and expanding awareness among healthcare providers and patients.
The development of hepatic fibrosis biomarkers continues to expand due to technological progress in the field. New methods for identifying and validating biomarkers benefit from the progress made in high-throughput screening, along with proteomics and genomics. The clinical adoption of diagnostic technologies such as advanced imaging techniques and multi-analyte blood testing continues to grow. The industry expands through government initiatives that promote liver disease awareness, fund biomarker investigations, and provide support for early detection initiatives. These developments together advance a dependable and patient-friendly approach to diagnosing liver conditions.
Product Insights
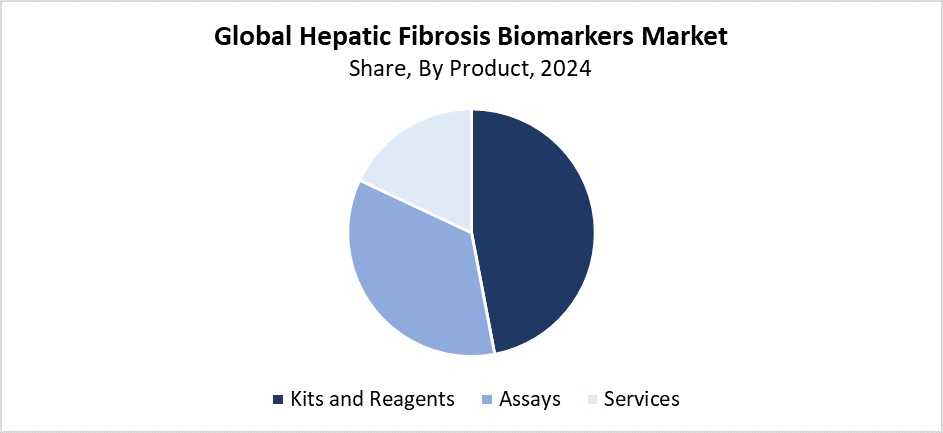
The kits and reagents segment held the largest revenue share of 47.52% and dominated the hepatic fibrosis biomarkers market. The market leadership of this segment exists because healthcare providers require straightforward and dependable liver fibrosis diagnostic tools that come at affordable prices. The practicality of diagnostic kits and reagents enables their widespread use in clinical laboratories as well as hospitals and research facilities. The expanding use of biomarker-based kits emerged as a result of increasing patient demand for medical tests that replace traditional biopsy procedures. The continuous enhancement of assay technology and biomarker discovery has made these products more reliable while also expanding their application for liver disease diagnosis.
The assays segment of the hepatic fibrosis biomarkers market is anticipated to grow at the fastest CAGR throughout the forecast period. The rising demand for accurate, sensitive, and non-invasive diagnostic methods to detect and monitor liver fibrosis progression drives this rapid market expansion. Assays provide better disease staging and therapy monitoring through their high-precision biomarker level measurements. Assay technologies advance their precision through ongoing developments, which include multiplex assays and high-throughput platforms. The growing interest in precise medical approaches, such as personalized medicine, drives the adoption of advanced assays that deliver detailed liver pathology understanding. These factors create strong market conditions that will drive significant growth in the assays market during the upcoming years.
Technology Insights
The immunoassay segment led the hepatic fibrosis biomarkers market in 2024 with the largest revenue share of 43.52%. The leadership position of immunoassays stems from their excellent sensitivity and specificity in detecting protein-based biomarkers linked to liver fibrosis. The testing methods of immunoassay, including chemiluminescence assays and ELISA, find frequent applications in clinical diagnostics due to their fast, accurate, and budget-friendly testing capabilities. Early diagnosis, along with disease monitoring, depends on these instruments that can detect extremely small amounts of fibrosis biomarkers present in blood samples. The segment maintains its market leadership through continuous technical improvements of immunoassay platforms, which incorporate automated processes with enhanced detection capabilities, leading to expanded laboratory adoption worldwide.
Over the course of the forecast period, the hepatic fibrosis biomarkers market's molecular diagnostics segment is anticipated to expand at the fastest CAGR. Technological developments like PCR, next-generation sequencing, and gene expression analysis, which offer extremely sensitive and specific identification of genetic markers linked to fibrosis, are the main drivers of this expansion. By providing comprehensive insights into the course of liver fibrosis, molecular diagnostics facilitate early diagnosis and individualized treatment. The growth of this market is also being fueled by an increase in research aimed at finding new molecular biomarkers and a growing need for accurate, non-invasive diagnostic instruments. The market for hepatic fibrosis biomarkers is predicted to develop significantly due to the increasing acceptance of these technologies in clinical settings as they become more widely available and reasonably priced.
Regional Insights
North America held the greatest revenue share of 47.36% in 2024, dominating the market for hepatic fibrosis biomarkers. The region's sophisticated healthcare system, high incidence of chronic liver disorders, and emphasis on early detection and disease management are the main causes of this leadership. The market is growing because of the existence of top diagnostic firms, intensive research projects, and large expenditures on raising awareness of and treating liver disease. Additionally, the use of cutting-edge biomarker-based diagnostic techniques is supported by advantageous regulatory frameworks and reimbursement policies. North America is now the biggest and most significant regional market for hepatic fibrosis biomarkers, thanks to increased government funding and initiatives for liver disease research.
Europe Hepatic Fibrosis Biomarkers Market Trends
The European market for hepatic fibrosis biomarkers experiences rapid growth because of the increasing prevalence of chronic liver conditions such as hepatitis and nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) in the region. The growing awareness of early diagnosis and non-invasive testing methods among people drives the increasing demand for sophisticated biomarker-based diagnostics. European healthcare systems are already established, along with government initiatives supporting research funding for liver diseases contribute to accelerating market growth. Biotechnological companies collaborate with academic institutions to drive innovation in biomarker discovery processes. European markets for hepatic fibrosis biomarkers will exhibit continuous growth because of both an aging demographic and increasing lifestyle-related risk elements that will persist into the future.
Asia Pacific Hepatic Fibrosis Biomarkers Market Trends
The Asia Pacific region experiences significant market growth for hepatic fibrosis biomarkers because liver diseases, including hepatitis B and non-alcoholic fatty liver disease (NAFLD) continue to increase. The growing understanding of non-invasive testing methods and early diagnostic procedures drives the market toward biomarker-based solutions. The market grows because healthcare facilities improve primarily in China, alongside India and Japan. The advancement is faster because governments increase their financial support for medical investigations. The number of liver diseases continues to increase because of lifestyle-based risk factors, together with population aging. Hepatic fibrosis biomarkers find increasing clinical and research applications in the Asia Pacific region because of its expanding economy, combined with improved medical services.
Key Hepatic Fibrosis Biomarkers Companies:
The following are the leading companies in the hepatic fibrosis biomarkers market. These companies collectively hold the largest market share and dictate industry trends.
- GENFIT
- Pfizer Inc.
- AstraZeneca
- Prometheus Laboratories
- Exalenz Bioscience Ltd (Meridian Bioscience)
- BioPredictive
- Quest Diagnostics
- Labcorp
- Siemens Healthineers AG
- Bristol-Myers Squibb Company
- Others
Recent Developments
- In March 2025, Precision BioSciences' lead product PBGENE-HBV became the first in vivo gene-editing treatment for chronic hepatitis B to enter clinical trials in the US after receiving FDA permission (IND approval). In order to eradicate the viral reservoirs, cccDNA and integrated HBV DNA, and achieve a functional cure, PBGENE-HBV employs ARCUS meganuclease that is supplied via lipid nanoparticles.
- In November 2024, together, Arbutus Biopharma and Barinthus Bio revealed fresh preliminary findings from the IM-PROVE II Phase 2a clinical trial treating chronic hepatitis B. VTP-300, low-dose nivolumab, and imdusiran together showed higher rates of HBsAg reduction, suggesting improved therapeutic potential for a functional cure. These findings support the effectiveness of treating chronic HBV by combining RNA-targeted treatments with immune modulators.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Decision Advisor has segmented the hepatic fibrosis biomarkers market based on the below-mentioned segments:
Global Hepatic Fibrosis Biomarkers Market, By Product
- Kits and Reagents
- Assays
- Services
Global Hepatic Fibrosis Biomarkers Market, By Technology
- Immunoassay
- Molecular Diagnostics
- Proteomics/Metabolomics
- Imaging-based Biomarker Assessment
Global Hepatic Fibrosis Biomarkers Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Global |
| Pages | 189 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Sep 2025 |
| Access | Download from this page |
