Japan Anti-Malarial Drug Market
Japan Anti-Malarial Drug Market Size, Share, and COVID-19 Impact Analysis, By Drug Class, (Artemisinin-based Combination Therapies (ACTs), Quinoline-based Drugs, Other Antimalarials), By Distribution Channel (Hospital Pharmacy, Retail Pharmacy, E-commerce), and Japan Anti-Malarial Drug Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Japan Anti-Malarial Drug Market Insights Forecasts to 2035
- The Japan Anti-Malarial Drug Market Size Was Estimated at USD 1031.9 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 4.21% from 2025 to 2035
- The Japan Anti-Malarial Drug Market Size is Expected to Reach USD 1623.9 Million by 2035
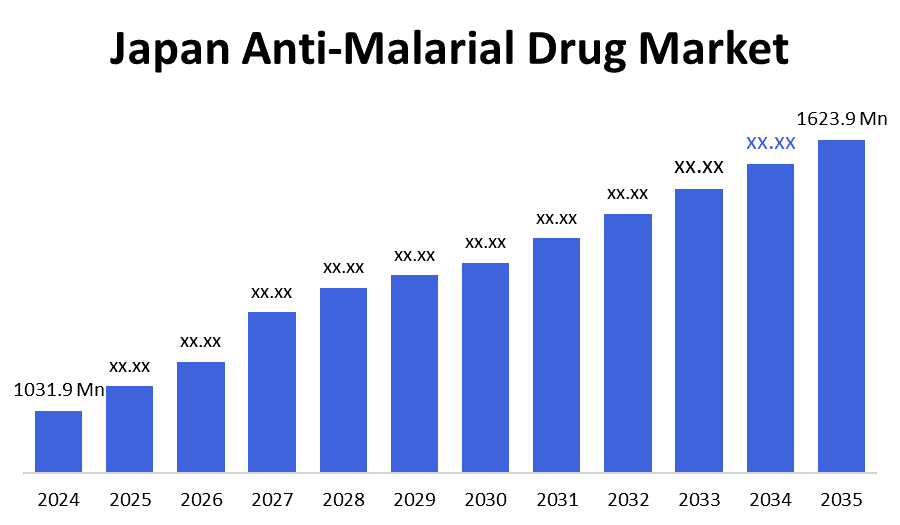
According to a research report published by decision advisor & Consulting, the Japan Anti-Malarial Drug Market size is anticipated to reach USD 1623.9 Million by 2035, growing at a CAGR of 4.21% from 2025 to 2035. Japan Anti-Malarial Drug Market is driven by the country's thriving pharmaceutical sector and its intensive R&D initiatives in drug discovery and vaccine development, as well as a dedicated effort to tackle drug-resistant malaria strains.
Market Overview
Antimalarial drugs are medicines used to treat or prevent malaria, a disease caused by Plasmodium parasites. They work by killing the parasites in red blood cells, and some can also protect people from getting malaria. These drugs come from natural sources, like quinine from cinchona bark, as well as from man-made chemicals. They are often used in combinations to make treatment more effective and to reduce the chance of drug resistance. Current trends in antimalarial drug development focus on combating resistance through the creation of novel drug classes and improved combination therapies. Antimalarial drugs are a varied group of medicines mainly recognised for their ability to destroy or stop the Plasmodium parasite from growing in the human body. Key trends include growing demand for artemisinin-based combination therapies (ACTs), heightened investment in research and development of new treatments such as triple combination therapies and long-acting injectables, and the appearance of drug-resistant strains, which drive the need for innovative solutions.
The Pharmaceuticals and Medical Devices Agency (PMDA), operating under the Ministry of Health, Labour and Welfare (MHLW), serves as Japan authority for drug approvals. The country offers several pathways to speed up the development and authorization of innovative therapies such as the SAKIGAKE Designation System and the conditional early approval framework although these programs are designed for the broader pharmaceutical sector rather than specifically for anti-malarial drugs.
Report Coverage
This research report categorizes the market for the Japan Anti-Malarial Drug Market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan Anti-Malarial Drug Market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan Anti-Malarial Drug Market.
Driving Factor
Japan Anti-Malarial Drug Market is driven by substantial R&D investment and innovation aimed at tackling global drug-resistant malaria rather than domestic disease burden. The market is propelled by strategic collaborations with global health organisations and the country’s advanced pharmaceutical infrastructure. Major factors driving growth include rising demand for advanced medical devices, greater government spending on modernising healthcare systems, and the creation of specialised tools like high-definition digital exoscopes and illuminated retractors.
Restraining Factor
Japan Anti-Malarial Drug Market is restrained by very low domestic malaria cases, stringent approval requirements, and unfavourable pricing rules linked to “drug lag” and “drug loss.” Japan’s anti-malarial market faces ongoing challenges.
Market segmentation
Japan Anti-Malarial Drug Market share is categorised by drug class and distribution channel.
By Drug Class
The Japan Anti-Malarial Drug Market is segmented by drug class into artemisinin-based combination therapies (ACTs), quinoline-based drugs, and other antimalarials. Among these, the artemisinin-based combination therapies (ACTs) are dominant in Japan Anti-Malarial Market due to their strong effectiveness and capacity to address drug-resistant strains, making them the treatment of choice for imported malaria cases in Japan.
By Distribution Channel
The Japan Anti-Malarial Drug Market is segmented by distribution channel into hospital pharmacy, retail pharmacy, and e-commerce. Among these, the retail pharmacy is dominant in Japan Anti-Malarial Drug Market due to large volume of prescription drug sales, the wide reach and strong consumer trust in established pharmacy chains, and the public’s inclination toward convenient, in-person health advice all contribute to this trend.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan Anti-Malarial Drug Market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Takeda Pharmaceutical Company Limited
- Eisai Co., Ltd.
- Daiichi Sankyo Company, Limited
- Mitsubishi Tanabe Pharma Corporation
- Shionogi & Co., Ltd.
- Astellas Pharma Inc.
- Chugai Pharmaceutical Co., Ltd.
- Otsuka Pharmaceutical Co., Ltd.
- Meiji Seika Pharma Co., Ltd.
- Kyowa Kirin Co., Ltd.
- Other
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
In May 2023: Eisai expanded its long-standing partnership with the GHIT Fund through a JPY 625 million contribution, bolstering the organisation’s next phase of research aimed at combating infectious diseases worldwide.
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. decision advisor has segmented the Japan Anti-Malarial Drug Market based on the below-mentioned segments:
Japan Anti-Malarial Drug Market, By Drug Class
- Artemisinin-based Combination Therapies (ACTs)
- Quinoline-based Drugs
- Other Antimalarials
Japan Anti-Malarial Drug Market, By Distribution
- Hospital Pharmacy
- Retail Pharmacy
- E-commerce
Frequently Asked Questions (FAQ’s)
Q: What is the Japan anti-malarial drug market size?
A: Japan Anti-Malarial Drug Market size is expected to grow from USD 1031.9 billion in 2024 to USD 1623.9 billion by 2035, growing at a CAGR of 4.21% during the forecast period 2025-2035.
Q: Who are the key players in the Japan anti-malarial drug market?
A: Key companies include Takeda Pharmaceutical Company Limited, Eisai Co., Ltd., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Other.
Q: How is the Japan anti-malarial drug market segmented by drug class?
A: The Japan Anti-Malarial Drug Market is segmented by drug class into artemisinin-based combination therapies (ACTs), quinoline-based drugs, and other antimalarials.
Q: What factors restrain the Japan anti-malarial drug market?
A: Japan Anti-Malarial Drug Market is restrained by very low domestic malaria cases, stringent approval requirements, and unfavourable pricing rules linked to “drug lag” and “drug loss,” Japan’s anti-malarial market faces ongoing challenges.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | country |
| Pages | 215 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
