Japan Cell-Based Immunotherapy Market
Japan Cell-Based Immunotherapy Market Size, Share, and COVID-19 Impact Analysis, By Therapy (Autologous Cellular Immunotherapy, Chimeric Antigen Receptor (CAR) T-Cell Therapy, Dendritic Cell-based Vaccine Therapy), By Primary Indication (B-cell Malignancies, Prostate Cancer, Renal Cell Carcinoma, Liver Cancer, Other), and Japan Cell-Based Immunotherapy Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Japan Cell-Based Immunotherapy Market Insights Forecasts to 2035
- The Japan Cell-Based Immunotherapy Market Size Was Estimated at USD 234.32 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 12.11% from 2025 to 2035
- The Japan Cell-Based Immunotherapy Market Size is Expected to Reach USD 823.8 Million by 2035
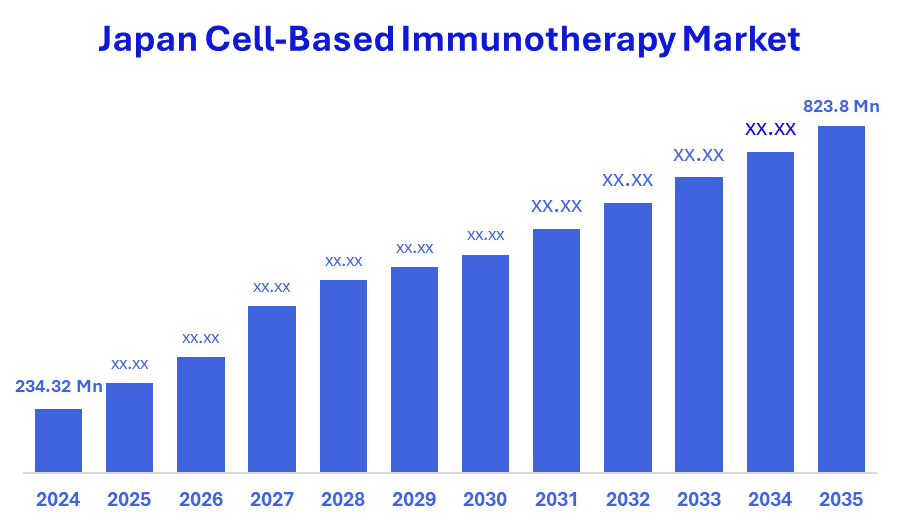
According to a research report published by Spherical Insights & Consulting, the Japan Cell-Based Immunotherapy Market size is anticipated to reach USD 823.8 Million by 2035, growing at a CAGR of 12.11% from 2025 to 2035. Japan Cell-Based Immunotherapy Market is driven by the rising incidence of cancer, rapid advancements in cellular therapy technologies, increasing demand for personalized and durable treatments, supportive government regulations and R&D funding, and an aging population, the market is experiencing significant growth.
Market Overview
Cell-based immunotherapy is a treatment approach that harnesses living immune cells typically derived from the patient to combat diseases such as cancer. The cells are extracted, genetically modified to improve their capacity to identify and destroy cancer cells, and then reintroduced into the patient’s body. Examples include Chimeric Antigen Receptor (CAR)-T and CAR-NK cell therapies, in which immune cells are engineered to specifically target and eliminate tumor cells. Major trends in cell-based immunotherapy include the advancement of allogeneic “off-the-shelf” therapies to lower costs and enhance accessibility, the extension of CAR T-cell treatments to solid as well as blood cancers, and the investigation of alternative cell types such as Natural Killer (NK) cells and Tumor-Infiltrating Lymphocytes (TILs) for solid tumor applications. Current innovations emphasize boosting cell durability, countering the immunosuppressive tumor environment, and optimizing manufacturing efficiency.
The Japanese government actively promotes cell-based immunotherapy as part of its broader “regenerative medicine” initiative, with data from the Ministry of Health, Labour and Welfare (MHLW) showing that 71,921 patients underwent regenerative medicine treatments in fiscal year 2020.
Report Coverage
This research report categorizes the market for the Japan Cell-Based Immunotherapy Market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan Cell-Based Immunotherapy Market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan Cell-Based Immunotherapy Market.
Driving Factor
The growth of Japan cell-based immunotherapy market is primarily driven by the rising cancer incidence and robust government initiatives, including accelerated approval programs like the Sakigake system and supportive reimbursement frameworks. This favorable landscape promotes technological progress in cell therapies and boosts demand for personalized, highly effective cancer treatment options.
Restraining Factor
Restraining factors for Japan cell-based immunotherapy market include the high treatment costs, intricate manufacturing procedures, and safety risks such as cytokine release syndrome. Additionally, the requirement for advanced infrastructure and the limited availability of therapies targeting specific diseases further impede broader market adoption.
Market Segmentation
The Japan Cell-Based Immunotherapy Market share is categorized by therapy and primary indication.
By Therapy
The Japan Cell-Based Immunotherapy Market is segmented by therapy into autologous cellular immunotherapy, chimeric antigen receptor (CAR) T-cell therapy, and dendritic cell-based vaccine therapy. Among these, the chimeric antigen receptor (CAR) T-cell therapy is dominant in the market for Japan Cell Based Immunotherapy in 2024, due to by several key factors, including its demonstrated high effectiveness in treating certain hematologic cancers, favorable regulatory support, and the rising demand for advanced and personalized cancer therapies.
By Primary Indication
The Japan Cell-Based Immunotherapy Market is segmented by primary indication into b-cell malignancies, prostate cancer, renal cell carcinoma, liver cancer, and other. Among these, the b-cell malignancies were the dominant indication in Japan's cell-based immunotherapy market in 2024 due to the proven efficacy and availability of approved cellular immunotherapies particularly chimeric antigen receptor (CAR) T-cell therapies that target specific markers such as CD19, these cancer types can be effectively treated.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan cell-based immunotherapy market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
The Key Companies
- Takeda
- Chugai
- Ono Pharmaceutical
- Novartis AG
- Gilead Sciences, Inc
- Bristol Myers Squibb Company
- bluebird bio, Inc.
- Adaptimmune Therapeutics plc
- Cellectis S.A.
- Other
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
In September 2022: Legend Biotech Corporation (NASDAQ: LEGN), a global biopharmaceutical company dedicated to developing, producing, and commercializing innovative treatments for serious illnesses, announced that Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved CARVYKTI (ciltacabtagene autoleucel). This chimeric antigen receptor T-cell (CAR-T) therapy targets the B-cell maturation antigen (BCMA) and has been authorized for adult patients with relapsed or refractory multiple myeloma. The approval applies to patients who have not previously received BCMA-targeted CAR-positive T-cell therapy and who have undergone three or more prior treatment lines—including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody with their disease showing resistance to or relapsing after the most recent therapy.
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Decision Advisor has segmented the Japan cell-based immunotherapy market based on the below-mentioned segments:
Japan Cell-Based Immunotherapy Market, By Therapy
- Autologous Cellular Immunotherapy
- Chimeric Antigen Receptor (CAR) T-Cell Therapy
- Dendritic Cell-based Vaccine Therapy
Japan Cell-Based Lmmunotherapy Market, By Primary Indication
- B-cell Malignancies
- Prostate Cancer
- Renal Cell Carcinoma
- Liver Cancer
- Other
Frequently Asked Questions (FAQs)
Q: What is the Japan Cell-Based Immunotherapy market size?
A: Japan Cell-Based Immunotherapy Market size is expected to grow from USD 234.32 million in 2024 to USD 823.8 million by 2035, growing at a CAGR of 12.11% during the forecast period 2025-2035.
Q: Who are the target audiences for this market report?
A: The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Q: Who are the key players in the Japan Cell-Based Immunotherapy Market?
A: Key companies include Takeda, Chugai, Ono Pharmaceutical, Novartis Ag, Gilead Sciences, Inc, Bristol Myers Squibb Company, Bluebird Bio, Inc., Adaptimmune Therapeutics Plc, Cellectis S.A., And Other.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | country |
| Pages | 224 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
