Japan Immunoprotein Diagnostic Testing Market
Japan Immunoprotein Diagnostic Testing Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Immunoassays, Radioimmunoassay, Immunoturbidimetry, and Others), By Application (Infectious Diseases, Oncology, Autoimmune Disorders, and Others), and Japan Immunoprotein Diagnostic Testing Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Japan Immunoprotein Diagnostic Testing Market Insights Forecasts to 2035
- The Japan Immunoprotein Diagnostic Testing Market Size Was Estimated at USD 387.4 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 7.59% from 2025–2035
- The Japan Immunoprotein Diagnostic Testing Market Size is Expected to Reach USD 865.9 Million by 2035
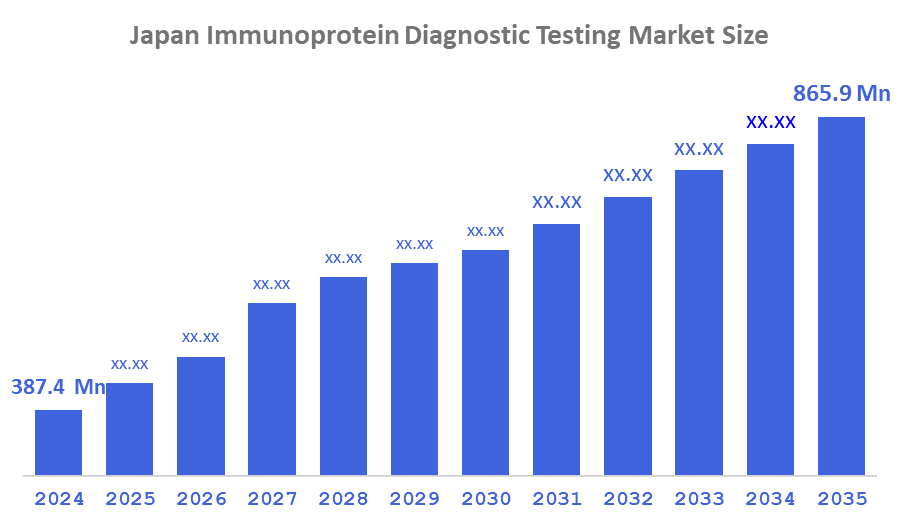
According to a research report published by Decision Advisor & Consulting, the Japan Immunoprotein Diagnostic Testing Market is anticipated to reach USD 865.9 million by 2035, growing at a CAGR of 7.59% from 2025–2035. The Japan immunoprotein diagnostic testing market is driven by the rising prevalence of chronic and autoimmune disorders, the expansion of cancer screening programs, and increasing use of highly sensitive immunoassay technologies in clinical laboratories. The demand for early and accurate diagnosis in infectious diseases, along with the growing adoption of high-throughput diagnostic platforms across hospitals and diagnostic centers, continues to boost market expansion in Japan.
Market Overview
The Japan immunoprotein diagnostic testing market refers to the specialized segment of diagnostic testing that measures immunoproteins, including immunoglobulins, complement proteins, acute phase proteins, and specific tumor or disease-related markers to help diagnose immune disorders, infectious diseases, inflammatory conditions, and cancer. Immunoprotein tests are widely used in clinical laboratories due to their ability to provide rapid, sensitive, and specific diagnostic results. Key trends shaping the market include the increased adoption of automated immunoassay analyzers, technological advances in chemiluminescence and immunoturbidimetric platforms, and rising demand for serum protein biomarkers for early disease detection. The market is also characterized by the growing need for precision diagnostics, expanded use of immunoproteins in oncology screening, and the rising incidence of lifestyle-related chronic diseases in Japan’s rapidly aging population.
National cancer screening programs, infectious disease surveillance reforms, and healthcare digitalization efforts further support the development of advanced immunoprotein testing capabilities across hospitals and reference laboratories in Japan.
Report Coverage
This research report categorizes the market for the Japan immunoprotein diagnostic testing market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan immunoprotein diagnostic testing market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan immunoprotein diagnostic testing market.
Driving Factor
The Japanese immunoprotein diagnostic testing market is driven by the increasing prevalence of autoimmune disorders, chronic inflammatory diseases, and cancers requiring immunoprotein-based biomarkers for accurate diagnosis and disease monitoring. Expanding use of high-sensitivity immunoassays, including chemiluminescent and fluorescence technologies, has enhanced detection capabilities, fueling industry growth. Rising healthcare spending, greater focus on preventive diagnostics, and wider adoption of automated laboratory instruments across hospitals and diagnostic networks further strengthen market expansion.
Restraining Factor
The Japanese immunoprotein diagnostic testing market faces restraints such as high operational costs associated with advanced immunoassay instruments, limited availability of skilled laboratory professionals, and challenges related to reimbursement for advanced biomarker tests. Additionally, complex regulatory pathways for new immunoprotein technologies may slow product adoption among diagnostic providers.
Market Segmentation
The Japan immunoprotein diagnostic testing market share is categorized by test type and application.
- The immunoassays segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan immunoprotein diagnostic testing market is segmented by test type into immunoassays, radioimmunoassay, immunoturbidimetry, and others. Among these, the immunoassays segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. Growth is attributed to the widespread adoption of chemiluminescent immunoassays, enhanced sensitivity for detecting disease-specific proteins, and broad applicability in screening infectious diseases, oncology markers, and autoimmune disorders. Automated immunoassay platforms reduce manual workloads and deliver rapid results, making them essential for high-volume hospital laboratories and diagnostic centers across Japan.
- The infectious diseases segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan immunoprotein diagnostic testing market is segmented by application into infectious diseases, oncology, autoimmune disorders, and others. Among these, the infectious diseases segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segment growth is driven by high demand for early detection of viral and bacterial infections, robust national disease surveillance programs, and increasing utilization of immunoprotein-based biomarkers for rapid and sensitive detection, particularly in community-based and hospital diagnostic settings.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within the Japan immunoprotein diagnostic testing market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Sysmex Corporation
- Fujifilm Holdings Corporation
- Roche Diagnostics Japan
- Siemens Healthineers Japan
- Tosoh Corporation
- Abbott Japan
- Thermo Fisher Scientific Japan
- H.U. Group Holdings
- Beckman Coulter Japan
- Shimadzu Corporation
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firms
- Venture Capitalists
- Value-Added Resellers (VARs)
Recent Developments
• In March 2025, Sysmex Corporation launched its latest high-sensitivity immunoassay analyzer designed to expand biomarker testing capabilities for autoimmune and infectious diseases across Japanese hospital laboratories.
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Decision Advisor has segmented the Japan Immunoprotein Diagnostic Testing Market based on the segments below:
Japan Immunoprotein Diagnostic Testing Market, By Test Type
- Immunoassays
- Radioimmunoassay
- Immunoturbidimetry
- Others
Japan Immunoprotein Diagnostic Testing Market, By Application
- Infectious Diseases
- Oncology
- Autoimmune Disorders
- Others
Frequently Asked Questions (FAQs)
Q: What is the Japan Immunoprotein Diagnostic Testing Market size?
A: The market is expected to grow from USD 387.4 million in 2024 to USD 865.9 million by 2035, at a CAGR of 7.59%.
Q: What factors restrain the Japan Immunoprotein Diagnostic Testing Market?
A: Key restraints include high equipment costs, regulatory challenges, limited skilled professionals, and reimbursement constraints.
Q: How is the market segmented by test type?
A: The market is segmented into immunoassays, radioimmunoassay, immunoturbidimetry, and others.
Q: Who are the key players in the Japan Immunoprotein Diagnostic Testing Market?
A: Major players include Sysmex, Fujifilm, Roche Diagnostics Japan, Siemens Healthineers, Tosoh, and others.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 180 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Dec 2025 |
| Access | Download from this page |
