Japan Immunotherapy Drug Market
Japan Immunotherapy Drug Market Size, Share, and COVID-19 Impact Analysis, By Therapy Type (Monoclonal Antibodies, Checkpoint Inhibitors, Cancer Vaccines, Cytokines, and Others), By Application (Cancer, Autoimmune Diseases, Infectious Diseases, and Others), and Japan Immunotherapy Drug Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Japan Immunotherapy Drug Market Size Insights Forecasts to 2035
- The Japan Immunotherapy Drug Market Size Was Estimated at USD 10125.6 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 10.31% from 2025 to 2035
- The Japan Immunotherapy Drug Market Size is Expected to Reach USD 29788.3 Million by 2035
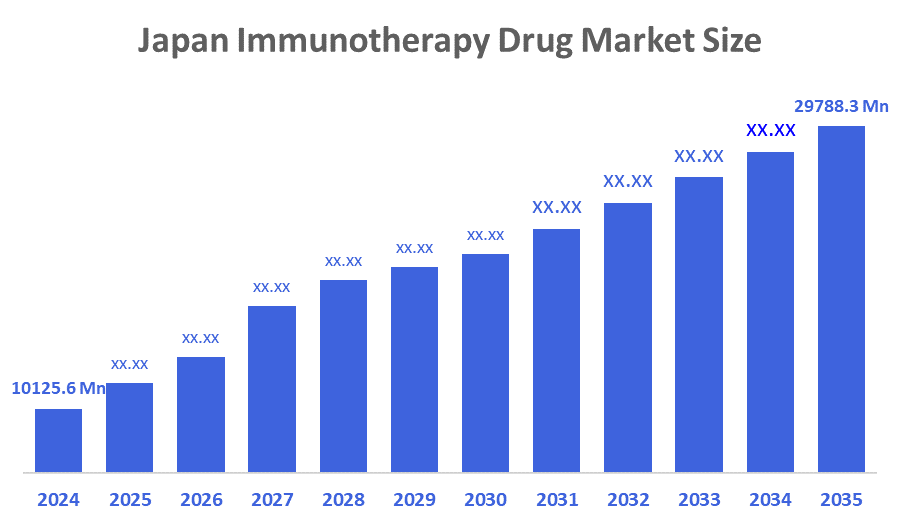
According to a Research Report Published by Decisions Advisors & Consulting, The Japan Immunotherapy Drug Market Size is anticipated to Reach USD 29788.3 Million by 2035, Growing at a CAGR of 10.31% from 2025 to 2035. The Japanese immunotherapy drug market is driven by the rising prevalence of cancer and autoimmune diseases, rapid advancements in biologics and genetic engineering, and increasing adoption of personalized targeted therapy. Strong investments in oncology research, expanded drug approvals, and the growing aging population further stimulate market growth.
Market Overview
The Japan immunotherapy drugs market refers to pharmaceutical treatments designed to activate or modify the body’s immune system to fight diseases such as cancer, autoimmune disorders, and chronic infections. Key forms include monoclonal antibodies, checkpoint inhibitors, cytokines, and cancer vaccines. The Japanese immunotherapy drug market is experiencing robust growth due to technological advancements in biologics, increased diagnosis of chronic diseases, and the accelerated regulatory approvals of advanced immune-targeting drugs. Japan is witnessing strong adoption of PD-1/PD-L1 inhibitors, CAR-T cell therapies, and novel monoclonal antibodies. Patients increasingly prefer immunotherapies owing to their targeted action and comparatively fewer long-term side effects than traditional chemotherapy. Japan imports a significant portion of advanced biologic components and hosts major clinical trials in oncology, autoimmune diseases, and cell-based therapy.
Government initiatives such as the AMED (Japan Agency for Medical Research and Development) funding programs, the Cancer Control Act, and national reimbursement frameworks support research, development, and commercial access to immunotherapy drugs. The Japanese government also accelerates advanced therapy approvals under its Sakigake Fast-Track Program, enhancing innovation and improving patient access to next-generation immunotherapies.
Report Coverage
This research report categorizes the market for the Japan immunotherapy drug market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan immunotherapy drug market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan immunotherapy drug market.
Driving Factors
The Japanese immunotherapy drug market is driven by rising cancer incidence, rapid advancement in biologics, and expanding investments into next-generation immunotherapies. The growing demand for targeted treatments, increased acceptance of PD-1/PD-L1 inhibitors, and strong R&D pipelines from global pharmaceutical companies further accelerate market growth. Japan’s aging population, high healthcare spending, and government support for clinical research enhance the adoption of innovative immune-targeted therapies across hospitals and specialty clinics.
Restraining Factors
The market is restrained by the high cost of immunotherapy drugs, which limits patient affordability even with insurance support. Strict regulatory requirements, complex biologics manufacturing processes, and limited access to advanced therapies in rural areas also restrict overall market penetration. High treatment costs, complex reimbursement, and stringent regulations.
Market Segmentation
The Japan immunotherapy drug market share is categorized by therapy type and application.
• The monoclonal antibodies segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan immunotherapy drug market is segmented by therapy type into monoclonal antibodies, checkpoint inhibitors, cancer vaccines, cytokines, and others. Among these, the monoclonal antibodies segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental growth is driven by strong adoption of antibody-based therapies in oncology and autoimmune conditions, rapid regulatory approvals, and increasing availability of biosimilars. Further advancements in antibody engineering enhance treatment precision and safety.
• The cancer segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
The Japanese immunotherapy drug market is segmented by application into cancer, autoimmune diseases, infectious diseases, and others. Among these, the cancer segment accounted for the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. The growth of the segment is due to rising cancer prevalence, expanded clinical trials, and increasing preference for immunotherapy over chemotherapy due to improved patient outcomes and targeted mechanisms of action. Rising cancer prevalence, increased R&D for advanced diagnostics, AI, immunotherapy, gene editing, more product approvals, better awareness, and pharmaceutical investment, with specific cancers driving key growth areas.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within the Japan immunotherapy drug market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Takeda Pharmaceutical Company Limited
- Ono Pharmaceutical Co., Ltd.
- Chugai Pharmaceutical Co., Ltd.
- Daiichi Sankyo Company, Limited
- Kyowa Kirin Co., Ltd.
- Bristol-Myers Squibb
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- AstraZeneca
- Other
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firms
- Venture Capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In March 2024, Ono Pharmaceutical announced the launch of a new PD-1 inhibitor for advanced cancer treatment in Japan. The therapy improves immune response against tumors and expands the company’s oncology portfolio.
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the Japan Immunotherapy Drug Market based on the below-mentioned segments:
Japan Immunotherapy Drug Market, By Therapy Type
- Monoclonal Antibodies
- Checkpoint Inhibitors
- Cancer Vaccines
- Cytokines
- Others
Japan Immunotherapy Drug Market, By Application
- Cancer
- Autoimmune Diseases
- Infectious Diseases
- Others
Frequently Asked Questions (FAQ’s)
Q: What is the Japan immunotherapy drug market size?
A: The market size is expected to grow from USD 10125.6 million in 2024 to USD 29788.3 million by 2035, growing at a CAGR of 10.31% during 2025–2035.
Q: What are the key growth drivers of the market?
A: Rising cancer incidence, expansion of biologics R&D, strong adoption of PD-1/PD-L1 inhibitors, a nd government support for advanced immunotherapy research.
Q: What factors restrain the Japan immunotherapy drug market?
A: High treatment costs, complex regulatory approvals, and limited access to advanced therapies in underserved regions.
Q: How is the market segmented by therapy type?
A: The market is segmented into monoclonal antibodies, checkpoint inhibitors, cancer vaccines, cytokines, and others.
Q: Who are the key players in the market?
A: Takeda, Ono Pharmaceutical, Chugai, Daiichi Sankyo, Kyowa Kirin, Merck, Bristol-Myers Squibb, AstraZeneca, Novartis, and Pfizer.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 257 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Dec 2025 |
| Access | Download from this page |
