Japan Pharmacovigilance Market
Japan Pharmacovigilance Market Size, Share, and COVID-19 Impact Analysis, By Type (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, EHR Mining), By Product Life Cycle (Pre-clinical, Phase I, Phase II, Phase III, Phase IV), By Process Flow (Case Data Management, Signal Detection, Risk Management System), By Therapeutic Area (Oncology, Neurology, Cardiology, Respiratory Systems, and Others), By End Use (Pharmaceuticals Companies, Biotechnology Companies, Medical Device Companies, and Others), and Japan Pharmacovigilance Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Japan Pharmacovigilance Market Insights Forecasts to 2035
- The Japan Pharmacovigilance Market Size Was Estimated at USD 7.22 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of Around 6.21% from 2025 to 2035
- The Japan Pharmacovigilance Market Size is Expected to Reach USD 14.01 Billion by 2035
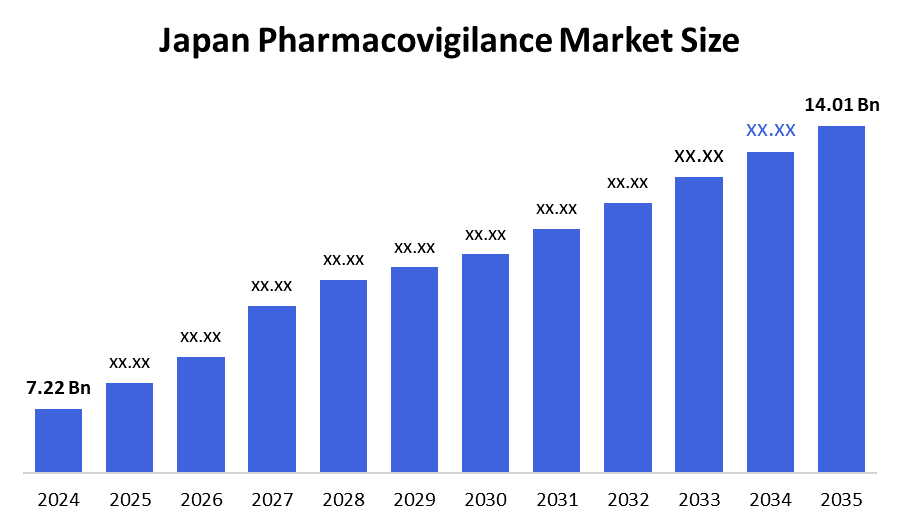
According to a research report published by decision advisor & Consulting, the Japan Pharmacovigilance Market size is anticipated to reach USD 14.01 Billion by 2035, growing at a CAGR of 6.21% from 2025 to 2035. The pharmacovigilance market is growing because there are more drugs and more people living with chronic diseases, which leads to more side effects that need tracking. Stronger government rules, outsourcing of safety monitoring, and the use of new technologies like AI and cloud systems are also boosting this market.
Market overview
Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products, and the World Health Organization (WHO) defines pharmacovigilance as the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. In Japan, the pharmacovigilance market is evolving with trends such as the integration of digital technologies, including AI for detecting adverse events, a stronger reliance on real-world data (RWD) to complement clinical trial findings, and greater outsourcing of PV functions to specialised service providers. The growth is fueled by a well-established pharmaceutical industry, strict PMDA regulations, and an aging population, with increasing attention on patient-centered approaches and personalized medicine. In the pharmacovigilance market, major factors affecting the pharmaceutical industry include increasing chronic diseases, stringent regulations, rising drug development, adverse drug reactions, and outsourcing. In addition, the adoption of technology, such as artificial intelligence, is becoming increasingly crucial.
Report Coverage
This research report categorizes the market for the Japan pharmacovigilance market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan pharmacovigilance market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan pharmacovigilance market.
Driving Factor
The Japanese pharmacovigilance market includes key driving factors include nation's aging population and the associated rise in chronic diseases, which increases drug consumption and adverse drug reactions (ADRs). Japan's mature regulatory system, overseen by the Pharmaceuticals and Medical Devices Agency (PMDA), enforces strict post-marketing surveillance and compliance requirements. Other factors include, increasing investments in pharmaceutical research and development, growing trend of outsourcing pharmacovigilance services by pharmaceutical companies to control costs and access expertise, Advancements in technologies like AI and cloud-based solutions, which enhance data analysis and reporting.
Restraining Factors
The Japan pharmacovigilance market faces several restraining factors, including stringent regulatory requirements, the high costs of ensuring compliance, and potential workforce shortages. Additionally, cultural nuances, linguistic diversity, and inconsistencies in adverse drug reaction reporting pose further challenges. Despite Japan’s highly developed healthcare system, these issues can hinder the market’s overall growth.
Market Segmentation
The Japan pharmacovigilance market share is categorized into type, product life cycle, process flow.
By Type
Japan pharmacovigilance market is segmented by type into, spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring and EHR mining. Among these, Spontaneous reporting is the dominant and foundational type of pharmacovigilance in Japan, due to its role in identifying new and rare adverse drug reactions (ADRs). The Japanese Adverse Drug Event Report (JADER) database is a key example of the spontaneous reporting system in action.
By Product Life Cycle
Japan pharmacovigilance market is segmented by product life cycle into, Pre-clinical, Phase I, Phase II, Phase III and Phase IV. Among these, phase IV pharmacovigilance, or post-marketing surveillance, is dominant in the Japanese market, similar due to the requirement for ongoing drug safety monitoring after approval and phase IV is crucial for detecting and assessing the long-term safety of drugs in a larger patient population, which is a key driver for the market segment.
By Process Flow
Japan pharmacovigilance market is segmented by process flow into, case data management, signal detection, risk management system. Among these, Signal detection holds the largest share of Japan’s pharmacovigilance market, as it is essential for identifying and managing possible drug safety risks. This segment leads the market because it is integral to a drug’s lifecycle, analysing data from sources like adverse event reports and clinical trials to spot safety issues.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan pharmacovigilance market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Otsuka Holdings Co. Ltd
- Parexel International Corporation
- Kyowa Kirin Co. Ltd
- Daiichi Sankyo Company, Limited
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent development
In May 2023: Labcorp and BML Research Institute’s joint lab for global clinical trials is boosting the pharmacovigilance market in the Asia-Pacific region, especially in Japan.
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2024 to 2035. decision advisor has segmented the Japan Pharmacovigilance Market based on the below-mentioned segments:
Japan Pharmacovigilance Market, By Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
Japan Pharmacovigilance Market, By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
Japan Pharmacovigilance Market, By Process Flow
- Case Data Management
- Signal Detection
- Risk Management System
Japan Pharmacovigilance Market, By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
Japan Pharmacovigilance Market, By End Use
- Pharmaceuticals Companies
- Biotechnology Companies
- Medical Device Companies
- Others
Frequently asked questions (FAQ’s)
Q: What is the Japan pharmacovigilance market size?
A: Japan Pharmacovigilance Market size is expected to grow from USD 7.22 billion in 2024 to USD 14.01 billion by 2035, growing at a CAGR of 6.21% during the forecast period 2025-2035.
Q: What factors restrain the Japan pharmacovigilance market?
A: The Japan pharmacovigilance market faces several restraining factors, including stringent regulatory requirements, the high costs of ensuring compliance, and potential workforce shortages. Additionally, cultural nuances, linguistic diversity, and inconsistencies in adverse drug reaction reporting pose further challenges. Despite Japan’s highly developed healthcare system, these issues can hinder the market’s overall growth.
Q: What is the recent development occurring in the Japan pharmacovigilance market?
A: Recent development occurring in Japan pharmacovigilance market in march 2023, Labcorp and BML Research Institute’s joint lab for global clinical trials is boosting the pharmacovigilance market in the Asia-Pacific region, especially in Japan.
Q: How is the Japan pharmacovigilance market segmented by product life cycle?
A: The Japan pharmacovigilance market is segmented by product life cycle into, Pre-clinical, Phase I, Phase II, Phase III and Phase IV.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | country |
| Pages | 171 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
