Japan Regulatory Information Management System Market
Japan Regulatory Information Management System Market Size, Share, and COVID-19 Impact Analysis, By Application (Registration, Submission, Publishing, and Others), By End-Use (Pharmaceutical, Biotechnology, and Clinical Research Organizations), and Japan Regulatory Information Management System Market Insights, Industry Trend, Forecasts to 2035
Report Overview
Table of Contents
Japan Regulatory Information Management System Market Insights Forecasts to 2035
- The Japan Regulatory Information Management System Market Size Was Estimated at USD 205.4 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 12.37% from 2025 to 2035
- The Japan Regulatory Information Management System Market Size is Expected to Reach USD 740.8 Million by 2035
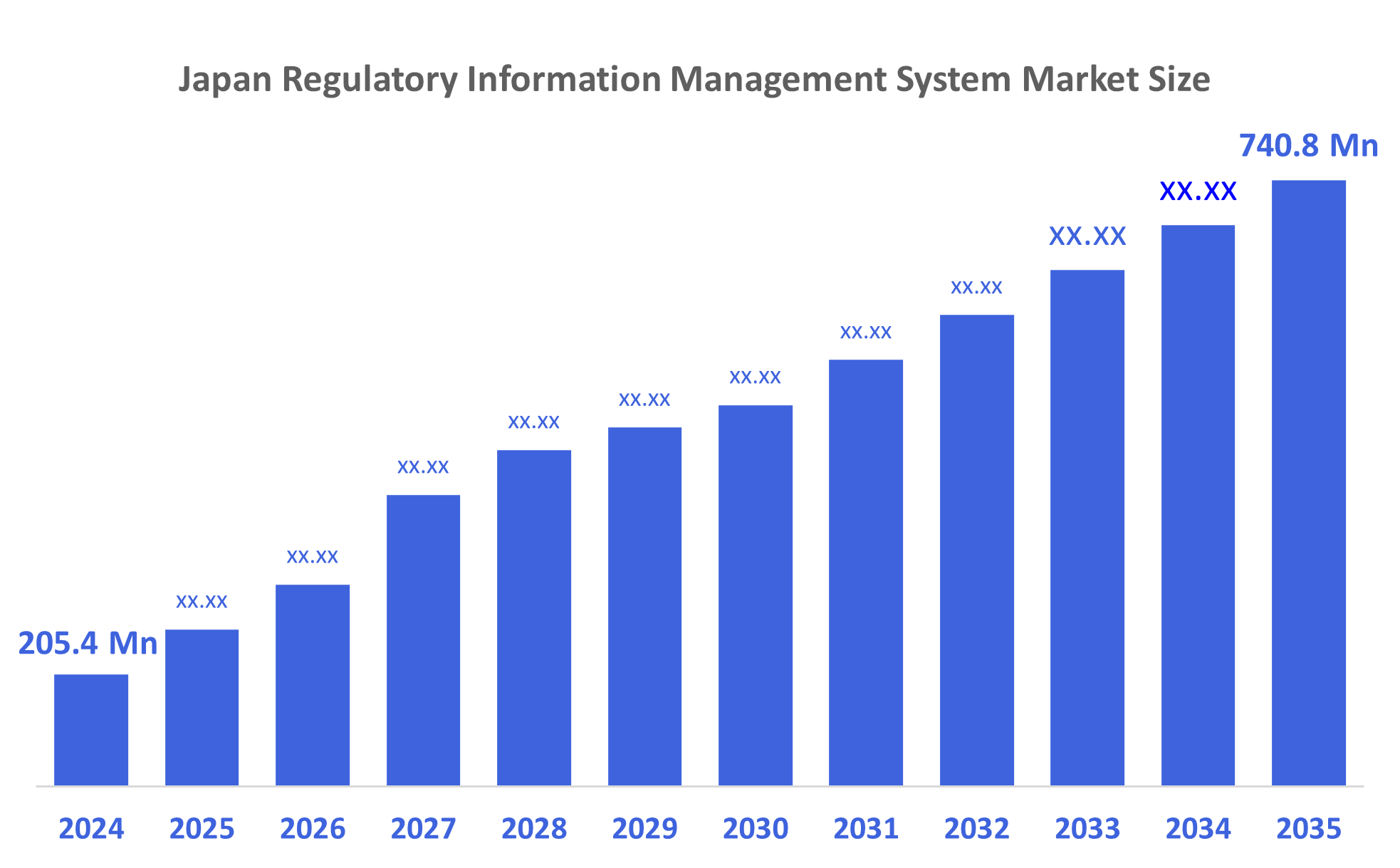
According to a research report published by Decisions Advisors, The Japan Regulatory Information Management System Market Size is Anticipated to Reach USD 740.8 Million by 2035, Growing at a CAGR of 12.37% from 2025 to 2035. The regulatory information management system market in Japan is driven by the increased investment levels, quick technical developments, and increased consumer demand for healthcare goods.
Market Overview
An integrated software platform called a regulatory information management system (RIMS) is used in highly regulated industries, including pharmaceuticals, biotechnology, medical devices, chemicals, and food and beverages, to centralize, organize, and streamline regulatory data, documentation, and processes. For managing product registration data, submission deadlines, compliance specifications, worldwide regulatory intelligence, and lifecycle management operations, it serves as a single source of truth. Regulatory information management system improves visibility, accuracy, and operational efficiency within regulatory affairs departments by replacing manual, fragmented, and spreadsheet-based processes. A regulatory information management system platform ensures that teams can instantly track submissions, approvals, renewals, variants, labelling amendments, and country-specific requirements by capturing the entire regulatory history of a product from manufacturing to post-marketing. To maintain consistency and reduce errors, it also interfaces with other enterprise systems, such as document management, quality management, ERP, and security databases. The ability of a regulatory information management system to provide real-time insight and analysis is one of its main benefits. Through dashboards and reports, regulatory teams can keep an eye on approval status, impending deadlines, resource burden, and compliance issues.
Report Coverage
This research report categorizes the market for the Japan regulatory information management system market based on various segments and regions, and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan regulatory information management system market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan regulatory information management system market.
Driving Factors
The regulatory information management system market in Japan is driven by demand for centralized data governance, increased pharmaceutical and medical device innovation, and stringent regulatory compliance requirements. Adoption has been further accelerated by the desire to reduce human errors and the growing digital transformation in the life sciences. The market growth momentum is further strengthened by government support and accelerated approval processes for digital health infrastructure.
Restraining Factors
The regulatory information management system market in Japan is mostly constrained by high implementation costs, difficult integration with current legacy systems, and a lack of regulatory IT knowledge in small businesses. Concerns about data security, drawn-out vendor evaluation processes, and resistance to workflow modifications hinder market adoption, particularly among pharmaceutical enterprises with traditional organizational structures.
Market Segmentation
The Japan regulatory information management system market share is classified into application and end-use.
- The registration segment dominated the market in 2024 and is expected to grow at a remarkable CAGR during the forecast period.
The Japan regulatory information management system market is segmented by application into registration, submission, publishing, and others. Among these, the registration segment dominated the market in 2024 and is expected to grow at a remarkable CAGR during the forecast period. The registration unit of RIMS is in charge of handling product registrations with different regulatory bodies and geographical regions. It involves keeping an eye on, updating, and maintaining the regulatory status of objects in order to ensure compliance with local, national, and international standards.
- The pharmaceutical segment dominated the market in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan regulatory information management system market is segmented by end-use into pharmaceutical, biotechnology, and clinical research organizations. Among these, the pharmaceutical segment dominated the market in 2024 and is expected to grow at a significant CAGR during the forecast period. This dominance is due to pharmaceuticals have a long and complex lifecycle, from early research and development (R&D) to clinical trials, approval processes, and market introduction. RIMS is crucial to managing this lifecycle since it guarantees that all regulatory criteria are met.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan regulatory information management system market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Veeva Systems
- ArisGlobal
- Freyr Solutions
- Rimsys
- AmpleLogic
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the Japan regulatory information management system market based on the below-mentioned segments:
Japan Regulatory Information Management System Market, By Application
- Registration
- Submission
- Publishing
- Others
Japan Regulatory Information Management System Market, By End-Use
- Pharmaceutical
- Biotechnology
- Clinical Research Organizations
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 220 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Nov 2025 |
| Access | Download from this page |
