Global Procalcitonin Test Market
Global Procalcitonin Test Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Rapid Test Kits, ELISA Test Kits, and Others), By Application (Chemiluminescence Immunoassay (CLIA), Enzyme-Linked Fluorescent Assay (ELFA), and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025-2035
Report Overview
Table of Contents
Global Procalcitonin Test Market Size Insights Forecasts to 2035
- The Global Procalcitonin Test Market Size Was Estimated at USD 460.66 million in 2024
- The Market Size is Expected to Grow at a CAGR of around 7.16 % from 2025 to 2035
- The Worldwide Procalcitonin Test Market Size is Expected to Reach USD 985.22 million by 2035
- Europe is expected to grow the fastest during the forecast period.
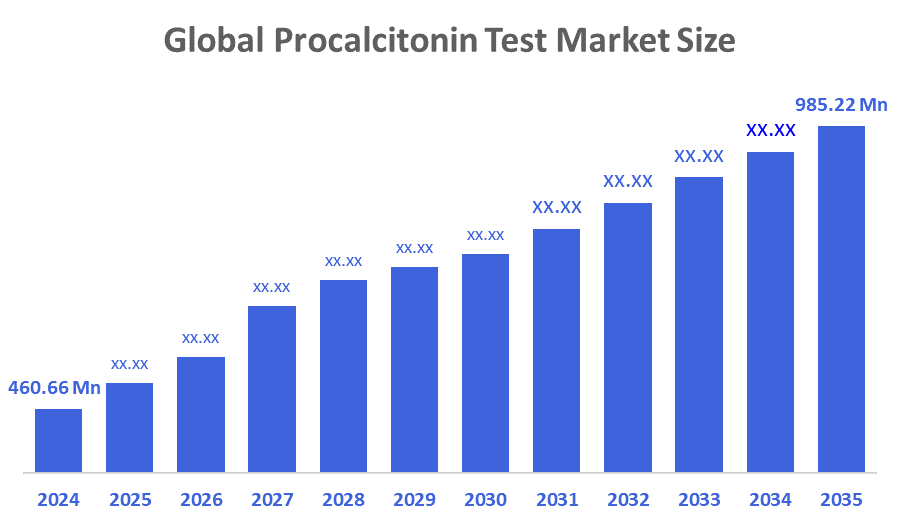
According to a research report published by Decisions Advisors and Consulting, The Global Procalcitonin Test Market Size Was Worth Around USD 460.66 Million In 2024 And Is Predicted To Grow To Around USD 985.22 Million By 2035 With A Compound Annual Growth Rate (CAGR) Of 7.16 % From 2025 To 2035. The rise of infectious diseases, such as septicemia, respiratory tract infections, and bacterial infections, has increased the need for procalcitonin testing because procalcitonin is a biomarker that can be used to evaluate the severity of these diseases. Additionally, technological advances in diagnostic equipment and laboratory automation have enhanced the efficiency and accuracy of procalcitonin testing, which has prompted more healthcare providers to use procalcitonin tests. Technological innovation also allows for a reduced turnaround time for procalcitonin tests, allowing for timely clinical decision-making.
Market Overview
The procalcitonin (PCT) test market refers to the global industry surrounding diagnostic assays that measure procalcitonin levels in blood or plasma, primarily used to detect and monitor bacterial infections, sepsis, and respiratory diseases. Procalcitonin (PCT) is a 116-amino acid protein that forms part of calcitonin. It is a hormone mostly secreted by parafollicular C cells in the thyroid gland, which is in charge of calcium homeostasis in the body. Procalcitonin is a fragment of preprocalcitonin produced by endopeptidase cleavage. Normal procalcitonin concentrations in adults are quite low, usually less than 0.1 ng/mL. An increase in procalcitonin above 0.25 ng/mL might be a sign of infection with bacterial origin, and in children, a PCT of over 0.5 ng/mL in those with urinary tract infections might indicate that the kidneys are involved. This is due to procalcitonin's quick reaction to bacterial infections; it has become an attractive diagnostic aid in many medical environments, such as primary care, emergency departments, and intensive care units (ICUs). Procalcitonin levels serve as a guide for doctors' clinical decisions, especially for the treatment of respiratory tract infections and sepsis. Studies have demonstrated that the employment of procalcitonin, directed protocols results in shorter courses of antibiotic therapy and lower death rates among critically ill patients with respiratory tract infections.
Report Coverage
This research report categorises the procalcitonin test market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the procalcitonin test market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub-segment of the procalcitonin test market.
Driving Factors
The growth of the market is largely attributable to the increased incidence of sepsis and bacterial infections, which have made the need for timely and accurate diagnostic tools imperative. The spreading knowledge and focus on early diagnosis and treatment of sepsis among healthcare professionals and establishments have substantially propelled the market growth. A major reason for the increasing growth of the procalcitonin test kit market is the escalating number of patients with sepsis and other serious bacterial infections worldwide. The rising awareness of the significance of early diagnosis and the function of procalcitonin as a biomarker for detecting bacterial infections and sepsis has resulted in a hike in the demand for procalcitonin test kits. The incorporation of point, of, care (POC) testing devices, which offer immediate answers, has also been a major driving factor for the market growth. Another key factor behind the market growth is the rising health care expenditure and better health care infrastructures in developing parts of the world. Governments and the private sector in these areas are making substantial investments in the health care sector so that the quality and accessibility of health care services can be improved.
Restraining Factors
The procalcitonin test kit market has attractive opportunities, but also has barriers that are preventing the market from growing. The dominant issue is that these advanced diagnostics, like procalcitonin testing, are generally expensive. However, the cost makes them hard to adopt, especially in low-resource environments. In addition, many healthcare providers do not know much about the use of procalcitonin test kits and how to interpret their results.
Market Segmentation
The procalcitonin test market share is classified into product type and application.
- The rapid test kits segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the product type, the procalcitonin test market is divided into rapid test kits, ELISA test kits, and others. Among these, the rapid test kits segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. Rapid test kits are anticipated to hold the majority of the market share because of their user, friendliness, fast results, and increasing utilization in point, of, care scenarios. Rapid test kits provide a number of benefits over the conventional methods since they can be utilised in different healthcare settings such as emergency rooms, intensive care units, and outpatient clinics, thus delivering immediate results which are vital for the prompt decision-making in sepsis treatment.
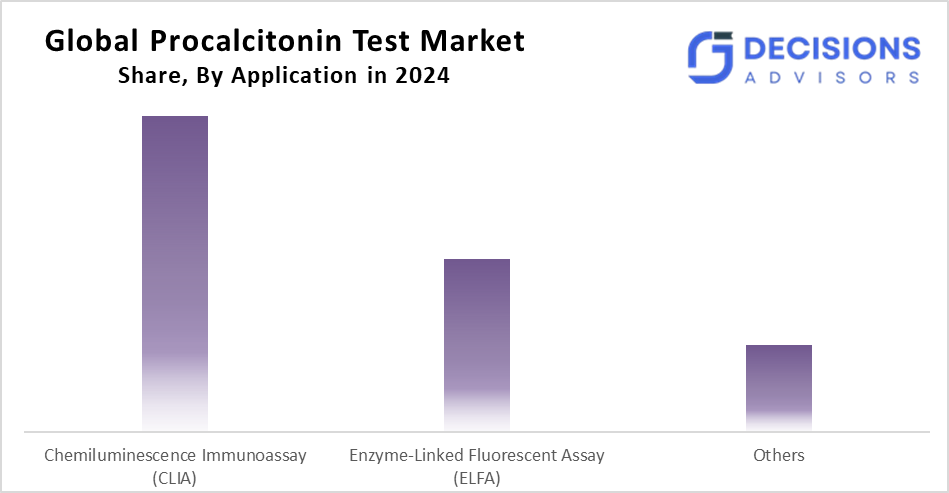
- The chemiluminescence immunoassay (CLIA) segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on the application, the procalcitonin test market is segmented into chemiluminescence immunoassay (CLIA), enzyme-linked fluorescent assay (ELFA), and others. Among these, the chemiluminescence immunoassay (CLIA) segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The segment growth is driven by its high sensitivity, specificity, and broad acceptance among clinical laboratories. The use of CLIA-based procalcitonin tests for measuring the amount of procalcitonin in a patient's sample through chemiluminescent reactions provides a rapid and precise quantification method. The automation capabilities of these tests mean that they can quickly process high volumes of samples, making them ideal for testing facilities such as hospitals and diagnostic labs. In addition to the above benefits, CLIA testing methods reduce sample handling and turnaround time, leading to more efficient workflows and more accurate diagnoses.
Regional Segment Analysis of the Procalcitonin Test Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
Asia Pacific is anticipated to hold the largest share of the procalcitonin test market over the predicted timeframe.
Asia Pacific is anticipated to hold the largest share of the procalcitonin test market over the predicted timeframe. The growing incidence of infectious diseases, the rise in knowledge related to sepsis management, and more developed healthcare facilities in the region are driving growth in this area. The major contributors are China and India, whose large patient base and focus on earlier diagnosis and treatment are driving their markets. The increase in healthcare spending, combined with new government programs to improve healthcare infrastructure, will continue to help drive future growth within this region.
Europe is expected to grow at a rapid CAGR in the Procalcitonin Test market during the forecast period. There are numerous opportunities for businesses in Europe due to the large number of people with sepsis and other bacterial infections, as well as the established healthcare systems across Europe. Countries such as Germany, France, and the United Kingdom are the largest markets in this region, due to the high number of advanced diagnostic capabilities and expenditures on healthcare. Research and development (R&D) is a focus in the region, and there are many leading-edge companies located there. As the public becomes more aware of early detection and the use of procalcitonin as a diagnostic tool for treating septic patients, this will drive growth in Europe's PCT market.
New UK-led research shows that procalcitonin (PCT) blood tests do not reduce antibiotic treatment duration in hospitalised children with suspected infections, challenging earlier hopes that biomarker-guided protocols could safely shorten therapy.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the procalcitonin test market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- Beckman Coulter, Inc
- Boditech Med Inc.
- DiaSorin S.p.A
- Getein Biotech, Inc.
- Radiometer Medical ApS
- Roche Diagnostics
- Shanghai Kehua Bio-engineering Co., Ltd
- Siemens Healthineers
- Snibe Diagnostic
- Thermo Fisher Scientific Inc.
- Wondfo Biotech
- Other
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In May 2025, Xiamen Wiz Biotech Co., Ltd, a global innovator in rapid diagnostic solutions, announced the launch of the next-generation Procalcitonin (PCT) Rapid Test Kit—a state-of-the-art in vitro diagnostic tool that delivers quantitative PCT test results in 15 minutes. Designed for hospitals, clinics, and laboratories, this bedside PCT test empowered clinicians to make evidence-based decisions for bacterial infection diagnosis, optimised antibiotic therapy, and helped combat the growing threat of antimicrobial resistance (AMR).
- In December 2024, A major UK trial (ADAPT-Sepsis) found that using a procalcitonin (PCT)-guided protocol safely reduced antibiotic duration in hospitalised patients with suspected sepsis, without increasing mortality. This supports biomarker-guided stewardship as a way to limit unnecessary antibiotic exposure.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the procalcitonin test market based on the below-mentioned segments:
Global Procalcitonin Test Market, By Product Type
- Rapid Test Kits
- ELISA Test Kits
- Others
Global Procalcitonin Test Market, By Application
- Chemiluminescence Immunoassay (CLIA)
- Enzyme-Linked Fluorescent Assay (ELFA)
- Others
Global Procalcitonin Test Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
- What is the current size of the global procalcitonin test market?
The market was valued at USD 460.66 million in 2024.
- What is the projected market size by 2035?
It is expected to reach USD 985.22 million by 2035.
- What is the CAGR for the forecast period?
The market is projected to grow at a CAGR of 7.16% from 2025 to 2035.
- What drives growth in the procalcitonin test market?
Rising cases of sepsis and bacterial infections, plus advances in point-of-care testing and healthcare infrastructure.
- Which product type leads the market?
Rapid test kits held the largest share in 2024 due to their speed and ease of use in emergencies.
- Which application segment dominates?
Chemiluminescence Immunoassay (CLIA) led in 2024, thanks to its high sensitivity and lab efficiency.
- Which region will hold the largest market share?
Asia-Pacific, driven by infectious diseases, healthcare spending, and growth in China and India.
- Who are the major companies in this market?
Key players include Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, and DiaSorin S.p.A.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Global |
| Pages | 240 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Feb 2026 |
| Access | Download from this page |
