Global Rare Musculoskeletal Disorder Treatments Market
Global Rare Musculoskeletal Disorder Treatments Market Size, Share, and COVID-19 Impact Analysis, Impact of Tariff and Trade War Analysis, By Treatment Type (Gene Therapy, Enzyme Replacement Therapy, and Small Molecule Drugs), By Disease Type (Duchenne Muscular Dystrophy, Spinal Muscular Atrophy, and Myasthenia Gravis), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Report Overview
Table of Contents
Rare Musculoskeletal Disorder Treatments Market Summary, Size & Emerging Trends
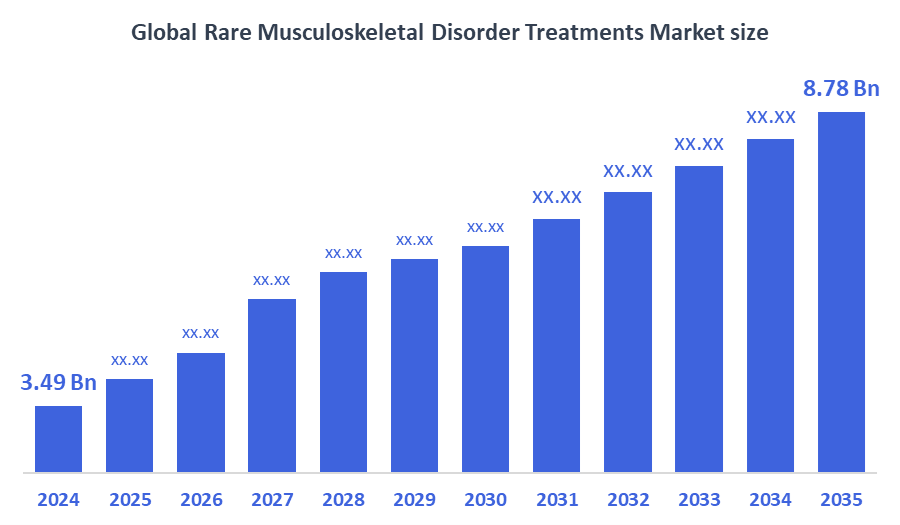
According to Decision Advisor, The Global Rare Musculoskeletal Disorder Treatments Market Size is Expected to Grow from USD 3.49 Billion in 2024 to USD 8.78 Billion by 2035, at a CAGR of 8.75% during the forecast period 2025-2035. Increasing advancements in gene and enzyme replacement therapies, rising awareness of rare diseases, and enhanced government support are driving significant growth in the market.
Key Market Insights
- North America is expected to account for the largest share in the rare musculoskeletal disorder treatments market during the forecast period.
- In terms of treatment type, the gene therapy segment dominated the market in terms of revenue during the forecast period.
- In terms of disease type, the duchenne muscular dystrophy segment accounted for the largest revenue share in the global rare musculoskeletal disorder treatments market.
Global Market Forecast and Revenue Outlook
- 2024 Market Size: USD 3.49 Billion
- 2035 Projected Market Size: USD 8.78 Billion
- CAGR (2025-2035): 8.75%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Rare Musculoskeletal Disorder Treatments Market
The rare musculoskeletal disorder treatments market focuses on the development and commercialization of therapeutic solutions targeting genetic and neuromuscular diseases with limited treatment options. These disorders, including Duchenne Muscular Dystrophy (DMD), Spinal Muscular Atrophy (SMA), and Myasthenia Gravis, require highly specialized treatment modalities due to their complexity and low prevalence. Gene therapy and enzyme replacement therapies have emerged as transformative approaches, offering potential disease-modifying effects. Additionally, small molecule drugs continue to play a critical role in symptom management and disease progression control. Regulatory support, orphan drug designations, and financial incentives for rare disease drug developers further enhance the market landscape.
Rare Musculoskeletal Disorder Treatments Market Trends
- Increasing adoption of gene therapy as a curative approach for rare neuromuscular disorders.
- Rising number of orphan drug designations granted by regulatory authorities worldwide.
- Growing partnerships between biotech firms and academic institutions to accelerate innovation.
- Increased use of AI and machine learning for drug discovery and patient diagnosis in rare disorders.
Rare Musculoskeletal Disorder Treatments Market Dynamics
Driving Factors: Growing R&D and Increasing Diagnosis Rates
One of the most significant drivers of the rare musculoskeletal disorder treatments market is the surge in research and development (R&D) investment from both public and private sectors. Governments, non-profit organizations, and pharmaceutical companies are increasingly funding projects aimed at developing novel therapies for rare diseases that historically received little attention due to their low prevalence. Improved genetic screening technologies, such as next-generation sequencing (NGS), whole genome sequencing (WGS), and newborn screening programs, have greatly enhanced the ability to identify rare musculoskeletal disorders early, often before symptoms appear. Early diagnosis enables timely intervention, which can significantly improve patient outcomes and support the case for more effective and targeted therapies.
Restrain Factors: High Treatment Costs and Limited Patient Population
Despite significant progress, the market faces serious headwinds, primarily related to cost and accessibility. Rare disease therapies especially gene and enzyme replacement therapies are among the most expensive treatments in the world, with single-dose gene therapies often costing over $2 million per patient. These high costs limit access, especially in countries lacking strong insurance or reimbursement systems. The small patient population for each rare musculoskeletal disorder further complicates matters. Because these diseases affect fewer individuals, the potential for economies of scale is minimal. As a result, manufacturers often set higher prices to recoup R&D investments, perpetuating affordability issues.
Opportunities: Advancements in Precision Medicine and Personalized Therapies
The rare musculoskeletal disorder treatments market is uniquely positioned to benefit from advancements in precision medicine and personalized therapies, which involve tailoring treatments based on a patient’s unique genetic makeup. These approaches offer higher efficacy and fewer side effects, especially important in disorders with diverse mutation profiles. The evolution of CRISPR-Cas9 and other gene-editing tools presents the possibility of single-dose curative treatments. Several biotech firms are actively exploring CRISPR-based therapies for conditions like Duchenne Muscular Dystrophy and other rare muscle disorders. Furthermore, next-generation gene delivery systems (e.g., AAV vectors) and RNA-based treatments (e.g., antisense oligonucleotides) are under development, broadening the therapeutic toolkit available for addressing the root causes of these diseases.:
Challenges: Regulatory Hurdles and Supply Chain Limitations
One of the persistent challenges in this market is the lengthy and uncertain regulatory approval process for rare disease therapies. Although mechanisms like accelerated approvals and orphan drug status exist, the high variability in patient responses and lack of standardized trial methodologies can slow down the process. There are also ethical and safety concerns, particularly with gene editing technologies, which are still relatively new and require long-term safety data. This uncertainty complicates trial design, consent processes, and regulatory evaluation.:
Global Rare Musculoskeletal Disorder Treatments Market Ecosystem Analysis
The ecosystem includes biotechnology firms, pharmaceutical companies, CROs (Contract Research Organizations), healthcare providers, patient advocacy groups, and regulatory bodies. Companies like Sarepta Therapeutics, Roche, Novartis, and Biogen lead innovation in this space. Academic research institutes and gene therapy developers play an integral role in drug discovery. Payers and insurers influence patient access, while governments offer financial incentives for orphan drug development. This ecosystem relies heavily on collaborative innovation and regulatory cooperation.
Global Rare Musculoskeletal Disorder Treatments Market, By Treatment Type
Gene therapy is currently the largest segment in the rare musculoskeletal disorder treatments market, accounting for approximately 52% of the global revenue in 2024. This dominance is largely due to breakthrough therapies that deliver corrective genes directly to patients suffering from diseases like Duchenne Muscular Dystrophy (DMD) and Spinal Muscular Atrophy (SMA). FDA-approved gene therapies such as Zolgensma have demonstrated significant long-term clinical benefits, effectively changing the treatment landscape and providing potentially curative outcomes. The promising results from early-stage gene therapy trials have encouraged increased investment and development in this space.
Enzyme replacement therapy (ERT) holds a significant portion of the market, comprising around 28% of revenue in 2024. ERT has a well-established role in treating the metabolic aspects of certain rare musculoskeletal conditions, such as those seen in Pompe disease or mucopolysaccharidoses, which affect muscle function indirectly. Its proven safety profile and effectiveness continue to make it a preferred option among clinicians and patients alike. However, ERT treatments typically require regular, lifelong administration, which can increase the treatment burden and costs.
Global Rare Musculoskeletal Disorder Treatments Market, By Disease Type
Duchenne muscular dystrophy (DMD) represents the largest disease segment in the rare musculoskeletal disorder treatments market, accounting for approximately 45% of the global revenue in 2024. This significant share is driven by the availability of advanced gene therapy options targeting the underlying genetic mutations responsible for DMD. The relatively large patient population, combined with increased awareness and early diagnosis through improved genetic screening, continues to fuel market growth. Ongoing research and clinical trials aimed at developing more effective treatments also contribute to the expanding market for DMD therapies. The segment is expected to maintain its leadership position, with steady growth projected through 2035 as new therapies emerge and patient access improves worldwide.
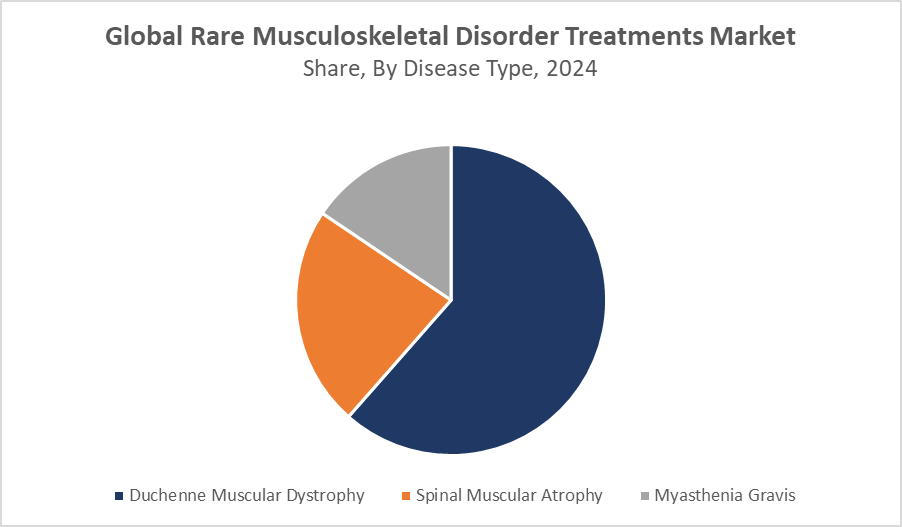
The spinal muscular atrophy (SMA) segment is experiencing rapid expansion, making up roughly 35% of the market revenue in 2024. This growth is largely attributable to recent approvals of breakthrough gene therapies such as Zolgensma and antisense oligonucleotide treatments like Spinraza, which have transformed the treatment landscape for SMA patients. These therapies have demonstrated significant clinical benefits, improving motor function and survival rates, which has led to widespread adoption globally. Increased newborn screening programs and earlier diagnoses are further driving demand.
North America is expected to hold the largest share of the rare musculoskeletal disorder treatments market throughout the forecast period.
This dominance is attributed to the region’s highly advanced healthcare infrastructure, widespread awareness of rare diseases, and strong regulatory frameworks that encourage innovation and rapid market access. Notably, the United States plays a leading role in this market, supported by a vibrant biotech ecosystem and active clinical trial programs focused on rare musculoskeletal disorders. Regulatory incentives such as the FDA’s Orphan Drug Act further stimulate the development and approval of novel therapies, enabling faster commercialization and broad patient access.
In contrast, the Asia Pacific region is forecasted to be the fastest-growing market for rare musculoskeletal disorder treatments.
This rapid growth is driven by improving diagnostic capabilities fueled by advances in genetic screening technologies, rising government healthcare expenditures, and expanding insurance coverage. The emergence of local biotech startups in key countries like China, Japan, and India is also accelerating innovation and treatment availability in the region. Furthermore, increasing patient awareness and government initiatives aimed at strengthening healthcare infrastructure are enabling greater access to rare disease therapies. These factors collectively position Asia Pacific as a dynamic and expanding market with significant untapped potential.
Europe represents a significant market for rare musculoskeletal disorder treatments due to its strong regulatory and healthcare frameworks.
The presence of centralized drug approval processes through the European Medicines Agency (EMA) facilitates efficient market entry for new therapies. Public healthcare systems across many European countries provide relatively broad access to rare disease treatments, supporting steady demand. Countries such as Germany and the United Kingdom are at the forefront of adopting innovative gene therapies for musculoskeletal disorders, supported by progressive reimbursement policies and investment in rare disease research. Europe’s collaborative environment, involving patient advocacy groups and government support, further strengthens its position as a key market in this space.
WORLDWIDE TOP KEY PLAYERS IN THE RARE MUSCULOSKELETAL DISORDER TREATMENTS MARKET INCLUDE
- Sarepta Therapeutics
- Novartis AG
- Biogen Inc.
- Roche Holding AG
- Pfizer Inc.
- Santhera Pharmaceuticals
- Ultragenyx Pharmaceutical Inc.
- Astellas Pharma Inc.
- Catalyst Pharmaceuticals
- Regenxbio Inc.
- Others
Product Launches in Rare Musculoskeletal Disorder Treatments Market
- In May 2024, Sarepta Therapeutics achieved a significant milestone with the FDA approval of its next-generation gene therapy for Duchenne Muscular Dystrophy (DMD). This approval marks a major advancement in the treatment options available for DMD, a rare and progressive muscle-wasting disorder. By expanding its product pipeline with this innovative therapy, Sarepta has reinforced its leadership position in the rare musculoskeletal disorder segment. The new gene therapy leverages cutting-edge technology designed to improve efficacy and safety, offering renewed hope for patients and caregivers.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Decision Advisor has segmented the rare musculoskeletal disorder treatments market based on the below-mentioned segments:
Global Rare Musculoskeletal Disorder Treatments Market, By Treatment Type
- Gene Therapy
- Enzyme Replacement Therapy
- Small Molecule Drugs
Global Rare Musculoskeletal Disorder Treatments Market, By Disease Type
- Duchenne Muscular Dystrophy
- Spinal Muscular Atrophy
- Myasthenia Gravis
Global Rare Musculoskeletal Disorder Treatments Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
FAQs
Q: What is the market size of the Global Rare Musculoskeletal Disorder Treatments Market in 2024?
A: The Global Rare Musculoskeletal Disorder Treatments Market size was USD 3.49 billion in 2024.
Q: What is the expected market size by 2035?
A: The market is projected to reach USD 8.78 billion by 2035.
Q: What is the forecasted CAGR for the Global Rare Musculoskeletal Disorder Treatments Market from 2025 to 2035?
A: The market is expected to grow at a CAGR of 8.75% during the forecast period 2025–2035.
Q: Which treatment type segment dominates the market?
A: Gene therapy dominates the market, accounting for approximately 52% of global revenue in 2024.
Q: What are the major disease types covered in the market?
A: The market covers Duchenne Muscular Dystrophy (DMD), Spinal Muscular Atrophy (SMA), and Myasthenia Gravis.
Q: Which disease segment holds the largest revenue share?
A: Duchenne Muscular Dystrophy accounts for the largest revenue share, around 45% of the market in 2024.
Q: Which region holds the largest market share?
A: North America is the largest market region in 2024.
Q: Which region is expected to grow the fastest during the forecast period?
A: Asia Pacific is expected to be the fastest-growing region in the market.
Q: What are the key drivers for market growth?
A: Increasing advancements in gene and enzyme replacement therapies, rising awareness of rare diseases, enhanced government support, and improved genetic screening technologies drive market growth.
Q: What challenges are limiting market growth?
A: High treatment costs, limited patient population, regulatory hurdles, and supply chain limitations are key challenges.
Q: What opportunities exist in the market?
A: Advancements in precision medicine, personalized therapies, and gene-editing technologies like CRISPR offer significant opportunities.
Q: Who are the key players in the Global Rare Musculoskeletal Disorder Treatments Market?
A: Leading players include Sarepta Therapeutics, Novartis AG, Biogen Inc., Roche Holding AG, Pfizer Inc., Santhera Pharmaceuticals, Ultragenyx Pharmaceutical Inc., Astellas Pharma Inc., Catalyst Pharmaceuticals, and Regenxbio Inc.
Q: What recent product launch has impacted the market?
A: In May 2024, Sarepta Therapeutics received FDA approval for its next-generation gene therapy for Duchenne Muscular Dystrophy, marking a significant advancement in treatment options.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Global |
| Pages | 255 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Oct 2025 |
| Access | Download from this page |
