South Korea Gene Therapy Market
South Korea Gene Therapy Market Size, Share, And COVID-19 Impact Analysis, By Therapy Type (Gene Augmentation, Gene Inhibition, Gene Editing [CRISPR, TALENs, ZFNs], Suicide Gene Therapy, Immune Modulation, Others), By Vector Type (Viral Vectors, Non-Viral Vectors), By Route of Administration (In Vivo, Ex Vivo, Intravenous, Intramuscular, Intrathecal, Sub-Retinal), By Application (Oncology, Rare Genetic Diseases, Neurological Disorders, Ophthalmic Diseases, Cardiovascular, Haematological, Infectious, Others), By End-User (Hospitals, Research Institutes, Specialty Clinics, Biotechnology & Pharmaceutical Companies), And South Korea Gene Therapy Market Insights, Industry Trends, Forecast To 2035
Report Overview
Table of Contents
South Korea Gene Therapy Market Insights Forecasts to 2035
- South Korea Gene Therapy Market Size Was Estimated at USD 6.72 Million in 2024.
- The Market is Expected to Grow at a CAGR of 4.87% from 2025 to 2035.
- South Korea Gene Therapy Market Size is Expected to Reach USD 11.34 Million by 2035.
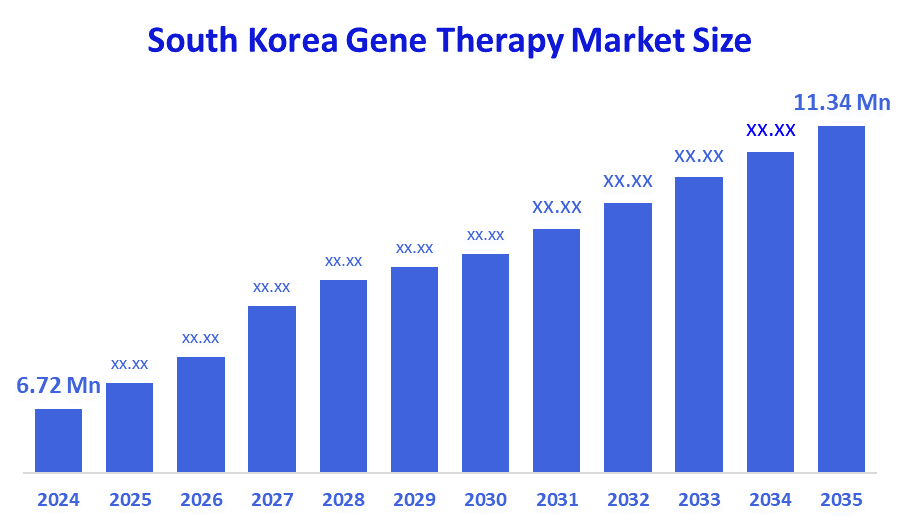
According to a research report published by Decision Advisor & Consulting, the South Korea Gene Therapy Market size is anticipated to reach USD 11.34 million by 2035, growing at a CAGR of 4.87% from 2025 to 2035. The South Korea gene therapy market is driven by rising cancer prevalence, increasing rare disease diagnosis, strong biotech investments, and government-backed bio health innovation programs strengthening advanced therapy development in the country.
Market Overview
Gene therapy refers to advanced medical treatments that modify or replace defective genes to treat diseases at their genetic root. In South Korea, gene therapy is rapidly adopted in key applications such as oncology, rare genetic disorders, ophthalmology, and neurological conditions, offering long-term and often curative therapeutic outcomes. These therapies use viral and non-viral vectors for in vivo and ex vivo delivery, enabling targeted and personalized treatment pathways. Moreover, the market growth is driven by rising cancer incidence, increasing awareness of inherited disorders, technological progress in vector engineering, and expanding clinical research infrastructure. Influencing factors include growing investments in cell and gene therapy manufacturing, expanding pipelines for CAR-T and CRISPR-based therapies, increased diagnostic capabilities, and strategic partnerships between domestic biotech firms and global innovator companies. Government initiatives such as the K-Bio Strategy, national cell-gene therapy regulations, and large-scale GMP facility expansion further accelerate market adoption. Future opportunities include scalable vector manufacturing, AI-powered genomic therapies, export-driven biotech growth, and South Korea’s progression toward becoming a leading APAC hub for advanced gene and cellular therapies.
Report Coverage
This research report categorizes the market for the South Korea gene therapy market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korea gene therapy market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the South Korea gene therapy market.
Driving Factors
The South Korea gene therapy market is driven by the growing prevalence of cancer and increasing adoption of gene-modified cell therapies, rising diagnosis of rare and inherited genetic disorders requiring curative treatments, technological advancements in viral vectors, genomic editing, and cell engineering, strong government initiatives supporting bio health innovation and GMP capacity expansion, and accelerating clinical trials along with partnerships between Korean biotech firms and global developers.
Restraining Factors
The South Korea gene therapy market is restrained by the high cost of gene therapy products and complex reimbursement pathways, limited local vector manufacturing capacity causing supply bottlenecks, regulatory complexities in advanced biologics development, and safety concerns related to long-term gene expression and immune responses.
Market Segmentation
The South Korea gene therapy market share is categorized by therapy type, vector type, route of administration, application, and end-user.
- The gene augmentation therapy segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea gene therapy market is segmented by therapy type into gene augmentation therapy, gene inhibition therapy, suicide gene therapy, immune modulation gene therapy, and genome editing technologies (CRISPR, TALENs, ZFNs). Among these, the gene augmentation therapy segment accounted for the largest revenue market share in 2024 and is expected to register a significant CAGR during the forecast period. This growth is supported by strong clinical adoption for monogenic disorders, rising demand for curative therapies targeting inherited diseases, increased investments in AAV-based augmentation programs, and favourable regulatory pathways encouraging development of long-term functional treatments.
- The viral vectors segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
The South Korea gene therapy market is segmented by vector type into viral vectors and non-viral vectors. Among these, the viral vectors segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. This dominance is attributed to the high efficiency and stable gene expression of viral vectors, increasing development of AAV and lentiviral platforms for both in vivo and ex vivo applications, strong investments in GMP vector manufacturing sites in South Korea, and widespread use of viral vectors in clinical trials for oncology, ophthalmology, and rare genetic disorders.
- The in vivo administration segment accounted for the largest revenue share in 2024 and is expected to maintain its dominance during the forecast period.
The South Korea gene therapy market is segmented by route of administration into intravenous, intramuscular, sub-retinal, intrathecal, in vivo, and ex vivo approaches. Among these, the in vivo administration segment accounted for the largest revenue share in 2024 and is expected to maintain its dominance throughout the forecast period. This is growth is fuels by rising adoption of direct gene delivery technologies, expanding development programs for ophthalmic and neuromuscular conditions, and growing availability of vector systems optimized for in vivo delivery with improved safety and targeting efficiency.
- The oncology segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea gene therapy market is segmented by application into oncology, neurological disorders, rare genetic diseases, ophthalmic diseases, cardiovascular diseases, haematological disorders, infectious diseases, and others. Among these, the oncology segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR over the forecast period. The segment's expansion is driven by rising cancer cases in South Korea, rapid clinical progress in gene-modified immunotherapies such as CAR-T and TCR-T treatments, increasing approvals of advanced cell-based therapies, and robust R&D investment focused on tumor-targeted vector engineering and personalized genetic oncology solutions.
- The hospitals segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
The South Korea gene therapy market is segmented by end-user into hospitals, specialty clinics, research institutes, and biotechnology & pharmaceutical companies. Among these, the hospitals segment dominated the market in 2024 and is projected to grow at a substantial CAGR throughout the forecast period. Growth is attributed to advanced healthcare infrastructure, rising establishment of cell and gene therapy centres within major hospitals, increasing patient access to specialized treatment facilities, and the expanding availability of comprehensive clinical support systems required for high-complexity gene therapy administration.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within The South Korea gene therapy market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Samsung Biologics
- Celltrion
- GC Pharma
- Kolon Life Science
- OliX Pharmaceuticals
- Helixmith
- SillaJen
- Pharmicell
- Medipost
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
• In October 2025, GC Cell, a South Korean biopharmaceutical company, received regulatory sandbox approval for AB?201, the first CAR?NK therapy targeting HER2+ solid tumours in Korea. This approval allows the company to test a novel immunotherapy approach in hard-to-treat solid tumors, supporting the development of innovative oncology treatments for patients.
• In March 2025, GC Cell initiated Phase?1 clinical trials for GCC2005, a CAR?NK therapy designed for T?cell lymphoma. This trial enables early testing of engineered immune cells to target cancer cells, advancing the translation of laboratory research into potential patient therapies.
Market Segment
This study forecasts revenue at the South Korea, regional, and country levels from 2020 to 2035. Decision Advisor has segmented the South Korea Gene Therapy Market based on the below-mentioned segments:
South Korea Gene Therapy Market, By Therapy Type
- Gene Augmentation
- Gene Inhibition
- Gene Editing (CRISPR, TALENs, ZFNs)
- Suicide Gene Therapy
- Immune Modulation
South Korea Gene Therapy Market, By Vector Type
- Viral Vectors
- Non-Viral Vectors
South Korea Gene Therapy Market, By Route of Administration
- In Vivo
- Ex Vivo
- Intravenous
- Intramuscular
- Intrathecal
- Sub-Retinal
South Korea Gene Therapy Market, By Application
- Oncology
- Rare Genetic Diseases
- Neurological Disorders
- Ophthalmic Diseases
- Cardiovascular
- Haematological
- Infectious
- Others
South Korea Gene Therapy Market, By End-User
- Hospitals
- Research Institutes
- Specialty Clinics
- Biotechnology & Pharmaceutical Companies
FAQ’s
Q. What is the projected market size & growth rate of the South Korea gene therapy market?
A. The South Korea gene therapy market was valued at USD 6.72 million in 2024 and is projected to reach USD 11.34 million by 2035, growing at a CAGR of 4.87% from 2025 to 2035.
Q. What are the key driving factors for the growth of the South Korea gene therapy market?
A. The South Korea gene therapy market is driven by rising cancer prevalence, increasing rare disease diagnoses, technological advancements in gene and cell therapies, strong government initiatives supporting bio health innovation, and expanding clinical research infrastructure.
Q. What are the major restraints in the South Korea gene therapy market?
A. The market is restrained by high therapy costs, complex reimbursement pathways, limited local vector manufacturing capacity, regulatory complexities in advanced biologics, and safety concerns related to long-term gene expression and immune responses.
Q. Which therapy types are covered in the South Korea gene therapy market?
A. The market is segmented by therapy type into gene augmentation therapy, gene inhibition therapy, suicide gene therapy, immune modulation therapy, and genome editing technologies (CRISPR, TALENs, ZFNs).
Q. Which vector types are covered in the South Korea gene therapy market?
A. The market is segmented by vector type into viral vectors (AAV, lentiviral, retroviral, adenoviral, HSV) and non-viral vectors (plasmid DNA, lipid nanoparticles, physical delivery methods).
Q. Which applications are covered in the South Korea gene therapy market?
A. The market is segmented by application into oncology, rare genetic diseases, neurological disorders, ophthalmic diseases, cardiovascular diseases, hematological disorders, infectious diseases, and others.
Q. Who are the top players operating in the South Korea gene therapy market?
A. Samsung Biologics, Celltrion, GC Pharma, Kolon Life Science, OliX Pharmaceuticals, Helixmith, SillaJen, Pharmicell, Medipost, Others.
Q. Which end-users are covered in the South Korea gene therapy market?
A. The market is segmented by end-user into hospitals, specialty clinics, research institutes, and biotechnology & pharmaceutical companies.
Check Licence
Choose the plan that fits you best: Single User, Multi-User, or Enterprise solutions tailored for your needs.
We Have You Covered
- 24/7 Analyst Support
- Clients Across the Globe
- Tailored Insights
- Technology Tracking
- Competitive Intelligence
- Custom Research
- Syndicated Market Studies
- Market Overview
- Market Segmentation
- Growth Drivers
- Market Opportunities
- Regulatory Insights
- Innovation & Sustainability
Report Details
| Scope | Country |
| Pages | 180 |
| Delivery | PDF & Excel via Email |
| Language | English |
| Release | Dec 2025 |
| Access | Download from this page |
